Osmosis Potato Lab: Salt Concentration & Mass Change
advertisement
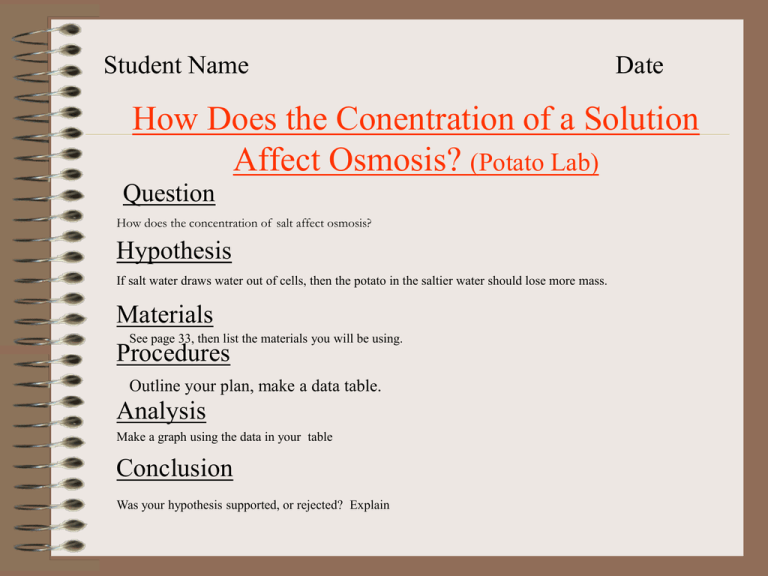
Student Name Date How Does the Conentration of a Solution Affect Osmosis? (Potato Lab) Question How does the concentration of salt affect osmosis? Hypothesis If salt water draws water out of cells, then the potato in the saltier water should lose more mass. Materials See page 33, then list the materials you will be using. Procedures Outline your plan, make a data table. Analysis Make a graph using the data in your table Conclusion Was your hypothesis supported, or rejected? Explain Question: How does the concentration of salts affect potatoes? Hypothesis: If salt water draws water out of cells, then the potato in the saltier water should lose more mass. Materials: After making a plan, list the materials you will need: _____________________________________ _____________________________________ _____________________________________ Procedures: You will be given two potato cubes, and two salt solutions: one that is 5% and one that is 10% salt. Plan how you will measure which potato loses more water over time. Check this plan with your teacher, then list the materials needed above, and list the procedures below. Procedures: To make sure you have a valid experiment planned, answer the following questions: 1. What variables will you attempt to control during the experiment? Size and shape of potato, temperature of solutions 2. What is the independent variable, ie. The thing you purposely change? The concentration of salt water, eg. 5% vs. 10% salt water. 3. What is the dependent variable, ie. the thing that you expect to change because of the independent variable. The mass of the potato cubes, after being in salt water solutions. Procedures cont’d: Copy the data table: Time (min) 0 2 4 6 8 10 Mass in 5% Mass in 10% Solution (g) Sol’n (g) Analysis: Make a graph as in (e) p. 33. Put mass on the side, or y-axis, and time on the bottom, or x-axis. Spread your numbers evenly along the length/height of the page. Include the units. Give your graph a descriptive title. Changing Mass of Potato in Different Concentrations of Salt Water 3.00 2.50 2.00 5% Sol'n 10% Sol'n Mass (g) 1.50 1.00 0.50 0.00 0 2 4 Time (min) 8 10 Conclusion: Was your Hypothesis supported, or rejected? Why should the potato in the 10% solution be lighter than the one in the 5% solution? My hypothesis was supported. The potato in the 10% solution should have been lighter because the cytoplasm has a higher concentration of water than the salt solution, so water osmossed out of the cell.