Reaction Type Flip Book
advertisement
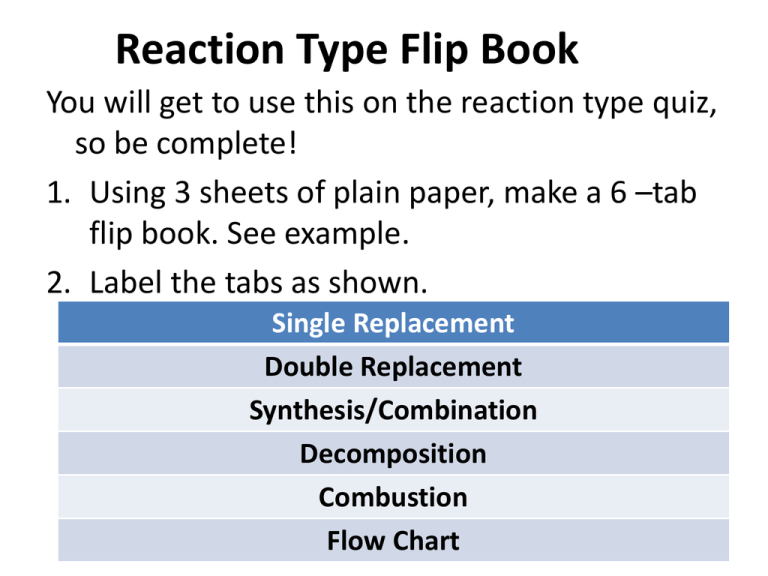
Reaction Type Flip Book You will get to use this on the reaction type quiz, so be complete! 1. Using 3 sheets of plain paper, make a 6 –tab flip book. See example. 2. Label the tabs as shown. Single Replacement Double Replacement Synthesis/Combination Decomposition Combustion Flow Chart Content • On each tab include the following… – Description of reaction type – At least one example of reaction type (chemical equation) from question 22 on page 224. • Flow Chart – The last tab will be a flow chart that will allow you to determine the reaction type. Reaction Type Flow Chart One reactant *Decomposition* 1. 2. 3. Break compound into two elements Two or more reactants More than 2 Elements 2 elements *Synthesis* 1. Combine elements Check for diatomic molecules 2. Balance Charges Balance equation 3. Balance Equation *Single Replacement* *Double Replacement* Element + Ionic Compound Two Ionic Compounds 1. Switch ions 1. Switch ions 2. Balance Charges 2. Balance Charges 1. Produces CO2 and H2O. 3. Balance Equation 3. Balance Equation 2. Balance Equation *Combustion* Oxygen + Carbon Compound on reactant side 1st Period: 2nd Period: 3rd Period: 1st Lunch: 4th Period: 4th Period: 2nd Lunch: Griz Period: 5th Period: 6th Period: 7:30-8:23 am 8:28-9:22 am 9:27-10:20 am 10:20-10:50 am 10:55 am-11:48 am 10:25 -11:18 am 11:18 am – 11:48 am 11:53 am – 12:15 pm 12:20 -1:13 pm 1:18 – 2:12 pm (53) (54) (53) (53) (53) (23) (53) (54) Grizzly Period moved to fifth period. All Freshmen in Focus and other interested Freshmen will go to the College and Career Fair during Grizzly Period with an assignment from their Focus teacher as a ticket back to class. Juniors will attend the Fair during 5th period with a completed worksheet as their evidence of attendance for your class. Seniors will attend during 6th Period. They too should have a completed worksheet. If nearly all of your class is a Junior or Senior group please plan to attend the fair with your entire class. If only a few are upperclass students then keep your group and send the individuals. Their absence should be treated like a field trip. Activity Series of Metals Lab Purpose: To determine which metals are more reactive. Procedure: Fill 5 wells with Copper Nitrate. Put 5 similar sized metals in each and observe at 1, 5 and 15 minutes. Record observations. Clean plates and repeat with 3 remaining solutions. * Lead Nitrate, Zinc Nitrate, Magnesium Nitrate Cu(NO3)2 Cu(NO3)2 Cu(NO3)2 Cu(NO3)2 Mg Cu Zn Pb Mg Copper Nitrate Lead Nitrate Zinc Nitrate Magnesium Nitrate Cu Zn Pb Activity Series Lab Questions 1. In which solutions did the appearance of the metals change? 2. Write balance equations for 6 of the reactions. 3. What type of reaction was illustrated in this lab? 4. Based on the results of your lab, write an activity series for the metals you tested. Rank/list them from most reactive to least reactive. Single Replacement Definition Single Replacement reaction – A single element takes the place of an element in a compound. A + BC B + AC Mg + 2HCl H2 + MgCl2 + + Double Replacement Definition Double Replacement reaction – Elements in 2 compounds switch places to form 2 new compounds. AB + CD AD + CB NaOH + HCl NaCl+ H2O + + Decomposition Definition Decomposition reaction – A single compound is broken down into 2 or more products. AB A + B H2O H2 + O2 + Synthesis Definition Synthesis reaction – 2 substances are combined to form a single product A + B AB Fe + O2 Fe2O3 + Combustion Definition Combustion reaction – Hydrogen or a hydrocarbon (H and C) burn in oxygen to produce water and carbon dioxide. Heat is given off as energy. C8H18 + O2 H2O + CO2 Quiz--Identify the reaction type represented here. + + + Identify the reaction type represented here. + + Identify the reaction type represented here. + + What type of reaction is this? O2 O2 O2 O2 H2O + CO2