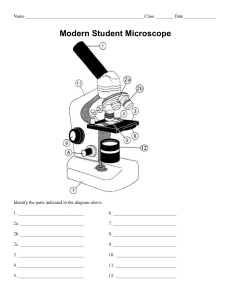
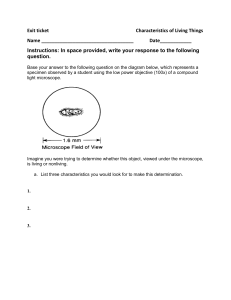
AUTOTECH 2MT3—STRUCTURE AND PROPERTIES OF MATERIALS EXPERIMENT #1 - MICROSCOPY A. Objectives 1. 2. 3. to learn how to prepare metallic samples for metallographic observation to learn how to use a metallurgical microscope and record micrographs to measure the grain size revealed in metallic samples B. Apparatus Cut off apparatus: Buehler Delta AbrasiMet Abrasive Cutter OR Leco SX100 Sectioning Machine Belt Sander: Metlab METS4SWB 4″ Metallurgical Benchtop Belt Sander Mechanical polishing platform: 1470A AB Handimet Grinder Polishing wheels: Polisher by Buehler Alumina suspensions: coarse (1 micron) and fine (0.05 micron) Etchants (ferric chloride and Nital) Microscopes: Zeiss Axiovert 25 CA Inverted Reflected Light Microscope and MetLab MET A0006 Upright Metallurgical Microscope C. Materials to be Examined Cartridge brass (70% Cu/30% Zn) Low carbon steel (1018) D. Procedure 1. Cutting of Samples 1.1 Clamp a brass strip in the vertical position in the cutter. Cut off a sample approximately 1” in length. Smooth off the edges on the belt sander. 260 brass strip Abrasive cutter 1.2 Obtain a steel sample from the instructor. These will have been pre-cut. Original cut samples of 260 brass and 1018 steel 2. Mechanical Sanding and Polishing 2.1 Grind any sharp edges on the samples using the belt sander. Be careful not to grind your finger tips. Belt Sander 2.2 Polish both samples on all grits from #240 to #600 for a few minutes at most on each grit or until the surface is completely covered by polishing scratches in one direction. Mechanical Polishing Platform 3. Polishing Wheels The polishing solutions will have been prepared using coarse and fine alumina powder. Coarse and fine alumina polishing powders Use the coarse polishing alumina first and polish so that all the scratches are removed from the 600 grit polished surface. Hold the sample on the polishing wheel and apply light pressure as you slowly rotate it in the direction opposite to the wheel’s motion. Wash the sample thoroughly with soap and water. Proceed to the fine polishing alumina. Polish until you have a mirror smooth surface. Wash again with soap and water, rinse with isopropyl alcohol and blow dry with compressed air. If you have water marks or stains repeat steps from cleaning wheel. You are ready for etching. 4. Chemical Etching The brass sample will be etched with ferric chloride solution (3.5g FeCl3, 50ml HCl, 100ml H20) etchant and steel with Nital etchant. The etching procedure is the same for both materials. Nital (left) and Ferric Chloride (right) etchants Etching Station Dip a cotton swab into the above etchant and then rub the cotton tip on a small area of the sample until a white haze appears or submerse the sample in the etchant using tongs. Rinse immediately in water and then alcohol. Go to the microscope and see if there is any structure. If you cannot see any structure you may have under etched. Repeat etching and make the area a bit whiter. Keep doing this until you can see structure. If the structure appears very dark, you have over-etched. In this case, go back to the fine polishing wheel for a minute or so to remove the etchant, then re-etch. 5. Microscope Use and Taking Photos Set up the Metlab microscope starting with 10x objective lens. Verify the quality of your polishing and etching with the instructor and then go to the Zeiss microscope for a photo. Metlab microscope Zeiss Microscope Take several photos of each sample at different magnification levels and save them to a USB flash drive. Make sure to add a scale bar to each photo. 6. Example: Grain size calculations Below is a photo of a steel grain structure. Print it out and complete the following (a) Using the scale bar, calculate the magnification of the photo using the formula MAG = (length of scale bar on photo)/(length of scale bar on sample) (b) Count the number of grains in the rectangle drawn on the photo (grains that cross the rectangle count as ½ grain). Measure the area of the rectangle and calculate the # of grains per square inch. (c) Convert the # of grains/in2 at the magnification (MAG) you calculated in (a) you calculated in (b) to the # of grains/in2 @ 100x magnification using the formula Grains/in2 @ 100x = N = [Grains/in2 @ MAG] x (MAG/100)2 (d) Calculate the ASTM grain size number G using the formula N = 2G-1 E. LABORATORY REPORT Abstract: This should be a brief (50-100 words) overview of what was done in the lab and what results were obtained. Introduction/Theory: Answer the following questions pertaining to microscopy: 1. A science microscope is shown below (you probably used one in high school biology class). Compare this with the metallurgical microscope shown on page 1. Would the biological microscope work for examining the metal samples? Why or why not? What do you see as the main difference between the two? Celestron binocular microscope 2. Why do metallic structures need to be etched before viewing? Procedure: Outline what was done in the lab, photos if necessary. Include a list of the equipment and materials used. Results: 1. Select one of your photos of steel for analysis. Import the photo into an editing program such as Paint. Calculate the magnification using the scale bar. Count the number of grains in a section of the photo and estimate the grain size number. See the attached worksheet in Section 6 for reference. Discussion: 1. Look at the photos of brass and steel on Avenue under “Lab 1”. What do you think is the best example for brass and for steel? Explain your answers. Compare the selected photos to the ones you recorded. Suggest how your photos could be improved. Be specific. 2. Below is an image of an automated polishing station. List some advantages and disadvantages of using the manual polishing method versus an automated set-up. Make suggestions about which technique to use in what industrial conditions. Take into consideration speed, level of expertise, cost, availability etc. 3. Identify any potential sources of error in the experiment. Conclusions: 1. Summarize briefly the steps in sample preparation. 2. Identify what factors are important in selecting a good quality image in metallography.