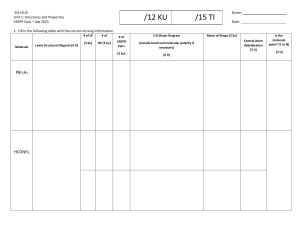
OBJECTIVES Determine if a molecule is polar or nonpolar given its structure (S11/12PSIIIc-15). the polarity of the bonds between the atoms 2the geometric shape of the molecule Two main factors determine the polarity of molecules. Polar or Non polar ? Electronegativity is the relative tendency of an atom to attract electrons to itself when chemically combined with other atoms. The higher the value of electronegativity, the more it tends to pull electrons toward itself Atoms and Electronegativity In a molecule of chlorine gas (Cl2), there is equal sharing of electrons which forms a bond between the two as in the case of hydrochloric acid (HCl) in figure 5, the shared electron tends to draw closer to the more electronegative atom which is chlorine. This bond is called polar covalent bond. A polar bond has a slightly negative end labeled with δ- (sigma), and a slightly THE DIFFERENCE OF ELECTRONEGATIVITY BETWEEN THE ATOMS FORMING A BOND CAN GIVE US AN INDICATION OF THE POLARITY OF THE BOND. THE GREATER THE The bonds between C (2.5) and O (3.5) in Figure 7 are polar each with an electronegativity difference of (/2.5 – 3.5/ =) 1.0 The water (H2O) molecule is another example of how the geometry of the molecule affects its polarity. While the bonds between H and O atoms are polar, its bent shape points the The Lewis electron dot diagram also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Valence Shell Electron Pair Repulsion (VSEPR) theory The VSEPR theory- is a guide for us to predict the arrangement of atoms in a polyatomic molecule. In the water (H2O) molecule, the central atom is oxygen since it is the least numerous element and can form more bonds than hydrogen. Create the appropriate Lewis dot structure of the molecule. 2. Using the Lewis structure as a guide, determine the appropriate VSEPR shape for the molecule. The number of electrons that are shared or bonded including the lone pairs will help determine the appropriate VSEPR shape