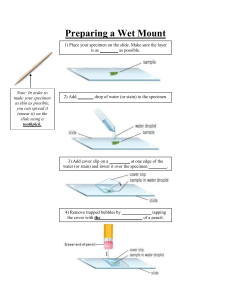
Medical Laboratory Report Patient UID No PRN No Registered On Patient Name : Mr AKARSHIT CHAWL Age and Gender : 24 Years / Male A Category : OPD - MEDIPLUS LAB (WAGHOLI) Referring Doctor : MEDIPLUS LAB Test Done : HOK210400174619 : HOK210400174619 : 13.09.2021 08:57 Sample UID No. A8174363 Observed Value COVID 19 RT PCR SPECIMEN COVID-19 QUALITATIVE PCR Target Genes ORF1 ab Gene N Gene RNaseP Gene NASOPHARYNGEAL / OROPHARYNGEAL SWAB NEGATIVE Detected / Not Detected CT Value Not Detected Not Detected Not Detected - Note : Interpreta on of the results: Speci c target gene considered for analysis of SARS COV-2 are ORF 1ab and N gene (covers N1 and N2 loci). Human RNaseP Gene serves as endogenous internal control gene. Test is considered posi ve if both the SARS COV-2 targets are detected. a) Note: • ICMR recommended kits are used for repor ng. All the specimen tes ng are no able to ICMR New Delhi and IDSP, Maharashtra State for further surveillance. • Invitrogen™ MagMAX™ RNA Isola on Kit along with automated RNA extractor is used. b) CLINICAL SIGNIFICANCE: • Clinical correla on with pa ent history, radiology ndings and co-infec on with other virus infec on is necessary to determine pa ent infec on status. • Samples with low viral load (CT 26 to 35) may give variable results on repeat tes ng. The possible reasons could be the varia ons in kits and instruments used. • Lower detec on limit of the assay is 10 GCE/Reac on. • Viral nucleic acid may persist in vivo independent of virus viability. Detec on of analy c target does not indicate that the viruses are infec ous or are the causa ve agents of symptoms. c) LIMITATIONS: • This test is a qualita ve assay and does not quan fy viral load. CT values are not an absolute indica on of viral load and are a ected by varia on in specimen collec on. • Op mal specimen types and ming of peak viral levels during infec ons of nCoV-19 have not been determined. Collec on of mul ple specimens is necessary in view of suspected clinical history. The repeat specimen may be considered a er a gap of 2-4 days a er the collec on of rst specimen for addi onal tes ng if required. • Nega ve results do not impede SARS - CoV - 2 infec on and should not be used as the sole basis for pa ent management decisions. Presence of inhibitors, muta ons and insu cient-viral RNA can in uence the result. d) METHODOLOGY: COVID-19 detec on by Polymerase Chain Reac on (PCR) is based on the ampli ca on of 2 speci c SARC-CoV-2 genes using Real Time PCR (Open System). In RT PCR, the ampli ed product is detected via uorescent dyes using CoviPath™ COVID-19 RT-PCR Kit along with CT cuto of kit recommenda on. e) DISCLAIMER: 1. This test is intended for use in conjunc on with clinical presenta on and other laboratory markers. 2. Improper specimen collec on, handling, storage and transporta on may result in false nega ve result 3. As per ICMR guideline CT value indicated in reports is not mandatory as well as advisable to be published on report it is men oned due to various enquiries received from Medical prac oners. 4. The report represents only the specimen received in the laboratory. ~~~ END OF REPORT ~~~ DR.SUMIT CHAVAN ( MD MICROBIOLOGIST ) Sample Collected On Results Authenticated : 13.09.2021 08:57 : 14.09.2021 16:27 Sample Accepted On : 13.09.2021 11:35 Results Reported : 14.09.2021 16:28 Page 1 of 1