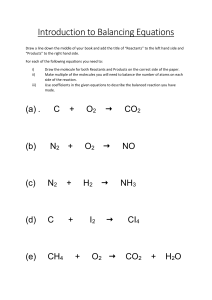
The total mass of the reactants ALWAYS equals the total mass of the products. The atoms just rearranged into different combinations. 1. The most reactive family of metals is called the alkali metals. 2. The most reactive family of nonmetals is the halogen family. Chemical Reactions are represented by equations. Chemical Equations show the reactants (starting substances) and the products (substances formed). Reactant 1 + Reactant 2 --> Product 1 Symbols used in equations: + Separates two or more reactants or products --> Separates reactants from products <--> Separates reactants from products and indicates a reversible reaction (s) Indicates a Solid state (l) " Liquid " (g) " Gaseous " Word Equations Use names of compounds and elements to indicate the reactants and products of chemical reactions: EX: Aluminum (solid) and Bromine (liquid) reaction word equation is: Aluminum (s) + Bromine (l) ->Aluminum Bromide (s) this reads as "aluminum and bromine react to produce aluminum bromide." Skeleton Equations: Uses chemical formulas instead of words to identify reactants and products. Ex: Al(s) + Br2(l) -> AlBr3(s) Write the skeleton equation for the reactions in these word equations: 1) Carbon (s) + Sulfur(s) -> Carbon Disulfide (l) C(s) + S(s) -> CS2(l) 2) Hydrogen (g) + Bromine (g) -> Hydrogen Bromide (g) H2(g) + Br2(g) -> HBr(g) 3)Carbon Monoxide(g) + Oxygen(g) -> Carbon Dioxide(g) CO(g) + O2(g) -> CO2(g) .