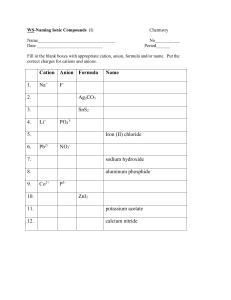
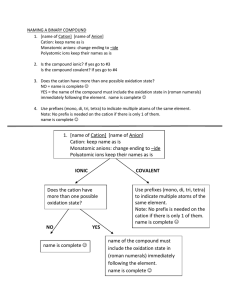
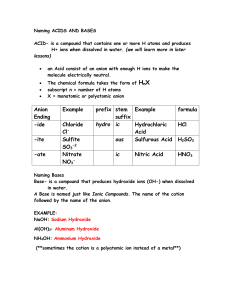
Chemical Nomenclature Naming Simple Chemical Compounds This worksheet will layout the basic rules for naming simple chemical compounds and assigning oxidation states to the various atoms. There are examples for each section on naming and deriving chemical formulas. Much of this information will need to be committed to memory, it is therefore good fodder for flashcards! Rules for Assigning Oxidation State ♠ Oxidation State (OS) of an individual atom in a free element is zero. ♠ Total OS of a neutral species is zero, for an ion it is equal to the charge on the ion. ♠ Group 1 metals are always +1, Group 2 metals are always +2. ♠ OS of Fluorine is -1 (This is usually true for all halogens). ♠ OS of Hydrogen is usually +1. ♠ OS of Oxygen is usually -2. ♠ In binary compounds: Group 17 → -1, Group 16 → -2, Group 15 → -3. Naming Binary Compounds Ionic Compounds: Metals and Non-metals Type 1: Metals that form only one cation (Alkali and Alkali Earth Metals, Groups 1 & 2). ♠ Cation is listed first, maintains it’s elemental name. Anion is listed second, it’s ending is changed to -ide. Example: CsBr (Cs+ , Br− ) → Cesium (cation) Bromide (anion, bromine changed to bromide) Type 2: Metals that form multiple cations (Usually transition metals). ♠ Cation is listed first, maintains it’s elemental name. The oxidation state of the cation is listed by roman numeral in parenthesis. Anion is listed last, it’s ending is changed to -ide. Example: CrCl3 (Cl is a Group 17 element and the anion is Cl− . If we have three of them, the cobalt must be Co3+ ) → Cobalt (cation) (III) (oxidation state of cation) Chloride (anion) Polyatomic Ions You are required to memorize the following list of polyatomic ions. There is only one cation on this list, all the others are anionic. Formula NH+ 4 NO− 3 CH3 COO− CN− OH− NO− 2 CO2− 3 O2− 2 SO2− 4 PO3− 4 Name ammonium nitrate acetate cyanide hydroxide nitrite carbonate oxide sulfate phosphate Type 3: Metals with polyatomic ions. ♠ Cation is listed first, with the oxidation state listed in parenthesis if the metal is capable of forming more than one ion. Polyatomic anion listed second with special name. Example: Ca2 CO3 → Calcium (cation) Carbonate (polyatomic anion name) Binary Covalent Compounds: Two Non-Metals The following list of numerical prefixes must be memorized. Prefix Number Prefix Number mono1 di2 tri3 tetra4 penta5 hexa6 hepta7 octa8 nona 9 deca10 Type 1: Two non-metals. ♠ First element in formula is listed first, using full elemental name. Second element is listed as an anion, with the ending changed to -ide. The numerical prefixes are used to denote the number of atoms present. Prefixes are used for both atoms in the compound with the exception that mono- is never used for the first element in the formula. Example: N2 O → dinitrogen (di- is required because we have two nitrogen atoms) monoxide (mono- is used because it is the second element in the formula) More Examples 1. For the following chemical formulas, give the name. a. ZnO b. CaCl2 c. AgF d. SrBr2 e. SnI4 f. CoCl2 2. For the following names, give the chemical formula: a. Strontium Nitride b. Potassium Sulfide c. Vanadium (II) Nitrate d. Sodium Cyanide e. Iron (III) Oxide f. Chromium (III) Acetate 3. For the following ion pairs write the chemical formula and the compound name: a. Rb+ and P3− b. Co3+ and F− 3. Cu+ and O2− 4. For the following chemical formulas, give the name: a. P2 O5 b. SiC c. BF3