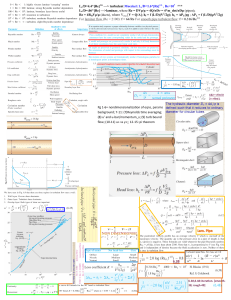
The SI unit for pressure is the pascal, Pa. Newtons are the product of mass (kg) multiplied with acceleration (meters per second per second squared). This is expressed as kg⋅m⋅s-2. By going back to the base units as above you can derive that a pascal is therefore: Joules are relevant to respiratory physiology and lung compliance. Question: Given that the joule is force x distance (which later becomes relevant to respiratory physiology and lung compliance), can you describe it in terms of base units? Answer: Force is the newton and is expressed as kg⋅m⋅s-2. The SI unit for distance is the metre, m. As joule is force x distance in SI units it must therefore be kg⋅m⋅s-2 x m. This is simplified to kgm2/s2. Flow Laminar Flow = Turbulent Flow= p= density Relation between Voltage and flow At low flows, how will the rotameter behave? Answer: At low flows, the bobbin is at the narrower end of the tube. This means that the gas flows across an obstruction of greater length than diameter, i.e. the gas will behave as if flowing through a tube and flow will be laminar. Question: At high flows, how will the rotameter behave? Answer: At high flows, the bobbin rises higher, into the wider portion of the tube. This means that the obstruction caused by the bobbin has greater diameter and reduced length, i.e. the gas behaves as if flowing through an orifice and flow is turbulent. Question: Why are rotameters gas specific? Answer: Because gases have different densities and viscosities, which in turn affect flow under different conditions. Question: What factors might affect the density or viscosity of a gas and have an effect on a rotameter's accuracy? Answer: The factors that might affect rotameter accuracy include: • Warmer gas has a lower density and viscosity, which may cause over-reading • Reduced atmospheric pressure reduces the density of a gas, which may cause over-reading (though viscosity is unaffected by pressure) No equation defines turbulent flow. However, Reynold's number (Re) can give an indication of when flow may be turbulent. Re is a dimensionless number (i.e. has no units) that can be calculated to help you to predict whether fluid flow is likely to be laminar or turbulent: • Re <2000 flow is likely to be laminar • Re >2000 flow is likely to be turbulent • ρ = fluid density • v = fluid velocity • d = tube diameter where: • η = fluid viscosity So, the critical velocity = Reynold's number = 2000. Transition from laminar to turbulent flow depends on the gases present. The Venturi effect: Based on the Bernoulli principle, where a drop in pressure (analogous to potential energy) is accompanied by a rise in flow (analogous to kinetic energy). Venturi masks use this principle to increase flow by entraining a second fluid into the flow path of the driving fluid. Logarithm Approximate Value Actual Value log 1 0 0 log 3 0.5 0.48 log 5 0.7 0.70 log 7 0.9 0.85 log 10 1.0 1.0 . Graham’s Law of Effusion Thomas Graham found that the rate of diffusion was inversely proportional to the square root of the molecular mass of the gas. So the larger the molecule the slower it diffuses. Effusion relates to the both the direction and the rate of change of diffusion. In our consideration this could be from alveolus to the plasma. So Rate of effusion is proportional to 1/Molecular mass This explains the second gas effect when using nitrous and a volatile in Oxygen Rate of effusion of A/Rate of effusion of B = Square root of molecular mass of B/Square root of molecular mass of A So if B is more massive than A, A will effuse out of the alveolus quicker than B, leaving behind more of B and so raising its concentration. Since, for example, halothane is more massive than nitrous oxide, Graham’s law will indicate that the nitrous will diffuse quicker and so raise the concentration of the halothane in the alveolus. Henry’s Law Henry’s law states that for a gas-liquid interface the amount of the gas that dissolves in the liquid is proportional to its partial pressure. So Henry’s law helps to predict how much gas will be dissolved in the liquid. The actual amount also depends on the solubility of the gas as well as its partial pressure. Dalton’s Law of partial pressure John Dalton observed that the total pressure of a gas mixture was the sum of the pressures of each of the gases if they were to exist on their own. Therefore P mixture = P1+P2+P3 + … This means that to calculate the total pressure in a cylinder for a mixture of gases just add up all the partial pressures. So if a cylinder of gas mixture at 400 kPa contains 21% oxygen and 79% helium, then if the oxygen existed on its own it would exert a partial pressure of 21% of 400 kPa = 88 kPa The helium therefore exerts a partial pressure of 400 – 88 = 312 kPa Capacitor T=R.C Current is proportional to rate of change of voltage Current is directly proportional to frequency CAPACITANCE (C): Is the ability of an object to store electrical charge. It is equal to the charge per unit voltage. It is measured in farads (Farad) Inductor T= L/R Voltage is directly proportional to rate of change of current Voltage is directly proportional to frequency Opposite of capacitor(henry) -------------------------------------------------------------------------------------------------------------------------------------- Fick’s law states that the rate of transfer across a membrane is proportional to the concentration gradient across the membrane and can be expressed as: Rate of diffusion across the cell membrane, Q= KpAC /T where C = concentration difference, A = area of membrane, Kp = Membrane pemeability T = membrane thickness Electrical Impedance Electrical impedance is directly proportional to frequency for an inductor (Z= 2 x Pi x f x L). Z: is the impedance in ohm L: is the value of the inductor in Henrys (H) Electrical impedance is independent of frequency for a resistance (Z= R). Electrical impedance is inversely proportional to frequency for a capacitor Z= 1/(2. Pi .f. C). The relative humidity is calculated from S.V.P. at dew point divided by S.V.P. at ambient temperature. --------------------------------------------------------------------------------------------------- The electromagnetic spectrum consists of radiation in ascending order of frequency as follows: radio waves, infrared, visible light, ultaviolet, x rays and gamma rays. Thus the wavelength of ultraviolet light is shorter than infrared as it has a higher frequency ------------------------------------------------------------------------------------ Power = VI= I2R Electric Energy = Power x Time