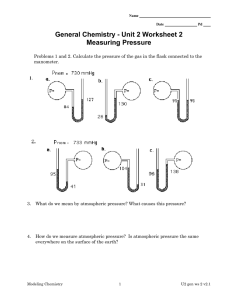
CHAPTER 1 INTRODUCTION TO CHEMISTRY AND MEASUREMENT •Introduction to quantitative chemistry •Measurement and Significant Figures •SI Units •Derived Units INTRODUCTION TO QUANTITATIVE CHEMISTRY WHAT IS CHEMISTRY ? Chemistry can be defined as the science that deals with the materials of the universe and the changes that these materials undergo. Ex: Air, Water, Rock, Animal, Gas, Cloth, Oil Concerned with composition, behavior/reaction, structure, properties of matter and the changes DEVELOPMENT OF MODERN CHEMISTRY Thousands before century : human used natural materials to make the useful products. Ex : fermenting beer and wine, extract chemicals from plants for medicine and perfume. Discovery of fire : develop ceramics, glass and metals. 18th century : Antoine-Laurent Lavoisier discover all materials composed of ‘atoms’ and can arrange into ‘molecules’. Development of instrument and apparatus. Ex : Battery, liquid-crystal display (LCD)-cell phone, computer, TV. EXPERIMENT AND EXPLANATION Experiment is an observation of natural phenomena carried out in a control manner to get the rational conclusions/result. Process of scientific method (method the scientist use to study the nature): • Observation – laws, hypothesis and theories • Conducting more experiments Experiments and their explanation depend on creativity and individually of researcher. MEASUREMENT AND SIGNIFICANT FIGURES Measurement is the comparison of a physical quantity to be measured with a unit of measurement (fixed standard of measurement) Length (m), Temperature (K), Time (s), Mass (g). Accuracy – how close the measured value is to the true or accepted value. Precision – how close a set of values obtained from measurement of quantity. Example??? RULES OF SIGNIFICANT FIGURES All non-zero digits are considered significant. Ex: 91, 234, 3.678 Zeros appearing anywhere between two non-zero digits are significant. Ex: 101.12 have five significant digits. Leading zeros are NOT significant. Ex: 0.00052 has two significant digits. RULES OF SIGNIFICANT NUMBER Trailing zeros in a number containing a decimal point are significant. Ex:12.2300 has six significant digits. The last significant digit of a number may be underlined; for example, 20000 has two significant digits. A decimal point may be placed after the number; Ex: "100.“ indicates three significant digits are meant. ROUNDING Rounding is the procedure of dropping non significant digits in calculation result or replacing it by another value that is approximately equal but shorter and simpler. If digit is greater or equal to 5, add 1 to the digit to be retained and drop all digits to the right. Ex: Rounding 1.2151 to three significant figures gives 1.22 If digit is less than 5, simply drop it and all the digit to the right. Ex: Rounding 1.2143 to three significant figures gives 1.21 EXERCISE Round off each of the following numbers to three significant figures. a) 63.351 e) 0.0020285 b) 0.0000004399 f) 90960 c) 10249000 g) 3.79745 d) 555.50 h) 7296.38 ANSWER Round off each of the following numbers to three significant figures. a) 63.351 = 63.4 b) 0.0000004399 = 0.000000440 e) 0.0020285 = 0.00203 f) 90960 = 91000 c) 10249000 = 10200000 g) 3.79745 = 3.80 d) 555.50 h) 7296.38 = 7300 = 556 MULTIPLICATION In multiplying two numbers, we have to find the least number of significant figures. This is the number of significant figures in the answer. Example : 0.024 x 1244 = 29.856 ≈ 30 DIVISION In dividing two numbers, the answer should contained the least number of significant figures from the two numbers. Example : 528 / 12.14 = 43.49259 ≈ 43.5 ADDITION AND SUBTRACTION In adding or subtracting, round to the least number of decimal places in the data. Example : 42.56 + 39.460 + 4.1 = 86.120 ≈ 86 SI UNIT (International System) International System of Units (SI) specifies a set of seven base units and also known as the metric system Divide into two: • Base Unit and Prefixes (Length, Mass, Temperature) • Derived unit ( Pressure, Volume, Density) SI BASE UNIT QUANTITY UNITS SYMBOL Length Meter m Mass Kilogram kg Time Second s Temperature Kelvin K Amount of Mole mol Ampere A Substance Electric current US UNIT QUANTITY UNITS Length Inch, Feet, Miles Mass Pound (lb), Ounce Temperature Fahrenheit (F) Volume Gallons SI PREFIX MULTIPLE PREFIX SYMBOL 1012 tera T 109 giga G 106 mega M 103 kilo k 10-1 deci d 10-2 centi c 10-3 milli m 10-6 micro µ 10-9 nano n UNITS CONVERSION Relationships of Some US & Metric Units 1 inch = 2.54 cm = 25.4 mm 1 lb = 0.4536 kg 1 mile = 1.609 km = 5280 ft 1 ft = 30.38 cm = 12 inch 1 gallon = 3.79 L 1 qt = 0.9464 L Temperature K = °C + 273.15 °C = K - 273.15 °C = (°F - 32) ÷ 1.8 °F = (°C × 1.8) + 32 DERIVED UNIT Derived by combination SI base units Ex 1: Velocity, v = Distance (m) = ms-1 Time (s) Ex 2: Acceleration, a = Velocity = ms-2 Time (s) DERIVED UNIT Ex 3: Volume, V • Cubic Volume • Cylinder Volume =Area x thickness = m2 x m = m3 = Area x height = r2 x m = m3 Ex 4: Density, d = Mass (kg) = kgm-3 Volume (m3) Density of water = 1000kgm-3 1 mL = 1 cm3 DERIVED UNIT Ex 5: Force, F = Mass (kg) x Acceleration (ms-2) = kgms-2 = Newton (N) Ex 6: Pressure, P = Force (N) = Nm-2 = Pascal (Pa) Area (m2) 1 atm = 101.325 kPa = 1.01325 Bar 1 atm = 14.7 psi 1 atm = 760 mmHg = 760 Torr EXERCISE 1. Pressure: 287.7 Psi Convert this pressure into kPa & Bar 2. Pressure: 55 mmHg Convert this pressure into Psi ANSWER 1. Pressure: 287.7 Psi Convert this pressure into kPa & Bar 14.7 psi = 101.325 kPa 1 psi = 101.325 kPa = 6.893 kPa 14.7 So, 287.7 psi = 287.7(6.893 kPa) = 1983.12kPa = 19.83 Bar ANSWER 2. Pressure: 55 mmHg Convert this pressure into Psi. 760 mmHg = 14.7 psi 1 mmHg = 14.7 psi 760 So, 55 mmHg = 55(14.7 psi) = 1.06 psi 760