6. Liquid/Liquid Extraction
advertisement

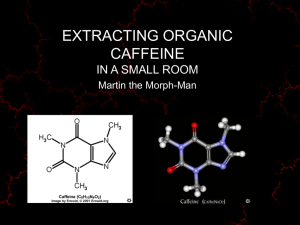
6. Liquid/Liquid Extraction PreLab : Prepare a PreLab as you have for the last two experiments and do this exercise: Draw a flow diagram similar to that in Figure 6.10 for the substances 2,4, and 6 shown in Figure 6.15. Introduction Extraction is the drawing or pulling out of something from something else. A lawyer extracts the truth from a criminal; athletes try to extract the last ounce of energy from their muscles. Chemists extract compounds from solids or liquids using an aqueous or organic solvent. By far the most universal and ancient form of extraction is the brewing of tea or the making of coffee. Every pot of coffee or cup of tea involves solid/liquid extraction, the extraction of organic compounds from solid ground beans or leaves using hot water as the liquid. The lower molecular weight polar molecules such as caffeine dissolve in the hot water and are removed from the high molecular weight water-insoluble cellulose, protein, and lipid materials. Over 200 compounds, some in only trace quantities, are extracted from the solid into a cup of coffee or tea. Decaffeinated coffee is also an excellent example of solid/liquid extraction. Coffee manufacturers extract the caffeine from the coffee to provide modern society with a decaffeinated version of an ancient drink. We will be demonstrating this chemical separation method in lab on a macroscale by extracting caffeine from tea. CH3 O CH3 O The brewing of tea is one form of extraction N N N N CH3 Figure 6.1: The Structure of Caffeine. Over the centuries, humans have carried out solid/liquid extraction by brewing just about every common plant leaf, fruit, or root. In the process, they have isolated a number of extracts with pharmacological activity. Many of these compounds were used for medicinal purposes. For example, Sertuner first extracted morphine from poppy seeds in 1805. This drug and several derivatives, including codeine, are used as pain-killers today. Unfortunately, other derivatives such as heroine, have become drugs of abuse. Legal and Illegal Drugs. O O O O H HO CH3CO CH3O HO H H Morphine N CH3 H HO H N H CH3 H CH3CO O H H Heroin Codeine Figure 6.2: Morphine, Codeine, and Heroin. 73 N CH3 Extraction will be used in many of the synthetic reactions. While solid/liquid extraction is the most common technique used to brew beverages and isolate natural products, liquid/liquid extraction is a very common method used in the organic laboratory. Organic reactions often yield a number of by-products, some inorganic, some organic. Also, since they do not go to 100% completion, some starting material is also often present at the end of an organic reaction. The real “work” in organic chemistry is not running the reaction, but rather in what is aptly called the “work-up” of the reaction mixture, that is, the separation and purification of the desired product from the mixture of by-products and residual starting material. Liquid/ liquid extraction is often used as the initial step in the work-up of a reaction, before final purification of the product by recrystallization, distillation or sublimation. A concrete example will help make sense of this. One of the synthetic reactions you will be carrying out this semester is a Grignard reaction involving the addition of phenyl Grignard reagent to benzophenone to form triphenylmethanol. MgCl HCl ether + MgCl O H2O OH + MgCl 2 phenylmagnesium chloride benzophenone triphenylmethanol The final reaction contains the product, the reaction solvents ether and aqueous hydrochloric acid, and probably traces of benzophenone starting material. Since water and ether are immiscible, we will have two separate layers, one aqueous acid, the other organic ether. Since ether is less dense than water, it will comprise the top layer. A novice might simply evaporate the water and ether to get rid of them. The problem is that the inorganic by-product, MgCl2, would not evaporate and the crystals of it would be mixed with crystals of the organic product triphenylmethanol. Your knowledge of chemistry and application of the principle like-dissolveslike should help you to figure out that in the 2-phase ether-water reaction mixture, the ionic inorganic salts of magnesium should want to be completely in the aqueous phase or layer, and the water-insoluble organic product, triphenyl methanol should want to be in the organic ether phase or layer. Extraction uses the solubility differences of these molecules to selectively draw the product into the organic layer. Although the two layers are immiscible, they work together to separate and select the compounds you are attempting to isolate. By simply separating these two layers, we can separate the inorganic salts from the organic materials. In almost all cases, extraction can be used to separate or “partition” ionic or polar low-molecular-weight substances into an aqueous phase and less polar water-insoluble substances into an immiscible liquid organic phase. This phenomenon is governed by the distribution coefficient. Distribution Coefficient K = distribution coefficient In the typical example of liquid/liquid extraction described here, the product was a fairly large organic molecule which you would predict to be not very soluble in water. On the other hand, if the product were a lower molecular weight or “small” molecule, you should predict that it might be at least partially water-soluble. Therefore, it might not completely “move” into the organic layer, but also partially dissolve in the aqueous layer. For water-soluble organic materials, such as acetic acid or sugar, most of the solute will reside in the water phase. A quantitative measure of the how an organic compound will distribute between aqueous and organic phases is called the 74 distribution or partition coefficient. It is the ratio, K, of the solubility of solute dissolved in the organic layer to the solubility of material dissolved in the aqueous layer. (Note that K is independent of the actual amounts of the two solvents mixed.) K= distribution coefficient K= solubility of organic (g/100 mL) solubility of water (g/100 mL) The constant K, is essentially the ratio of the concentrations of the solute in the two different solvents once the system reaches equilibrium. At equilibrium the molecules naturally distribute themselves in the solvent where they are more soluble. Inorganic and water soluble materials will stay in the water layer and more organic molecules will remain in the organic layer. By using the correct solvent system, a molecule can be specifically selected and extracted from another solvent. Since the distribution coefficient is a ratio, unless K is very large, not all of a solute will reside in the organic layer in a single extraction. Usually two, three, or four extractions of the aqueous layer with an organic solvent are carried out in sequence in order to remove as much of the desired product from the aqueous layer as possible. The effectiveness of multiple small volume extractions versus one large volume extraction can be demonstrated by a simple calculation. Imagine that one extraction can recover 90% of the compound. A second extraction with the same solvent may be able to pull out 90% of the remaining material. Effectively 99% of the compound was recovered with two extractions. One large extraction would have only obtained the initial 90%. Many smaller extractions are more efficient than one large extraction. This phenomenon can be proved mathematically, but in short follows the equation: 1 1 + VB VAn K fraction extracted into B = n This equation provides the fraction of material extracted by solvent B where n is the number of extractions performed, K is the distribution coefficient, VA is the volume of solvent A and VB is the volume of solvent B. There is a problem at the end of this chapter to demonstrate that more extractions are better than one larger extraction. Give it a try! Distribution coefficients play a large role in the efficacy of a drug. In order for a drug to be absorbed into a brain cell, it must pass through what is called the blood-brain barrier, into the brain cell. The drug must have enough water solubility to dissolve in the blood and be carried to the brain. However, to pass through the cell wall which consists largely of water insoluble fatty lipids with solubility properties similar to an organic solvent, the drug must have a reasonable organic solvent solubility too. Cell membranes use the same fundamental solubility principles as the extraction process. The cell membrane shown in Figure 6.4 consists of an ionic head and a very nonpolar or hydrophobic center. Ionic Head Nonpolar interior Ionic Head Figure 6.4: Simple schematic of a cell membrane. 75 Cell membranes use the same solubility principles as extraction. The ionic head of the lipid orients itself in aqueous environments creating a very nonpolar interior. Ions such as K+ and Ca+ can not traverse the interior of the cell readily because the interior is very nonpolar and will not support these ions. Extraction uses this same partitioning effect to isolate organic compounds. Just like this biological example, in the extraction process organic compounds will choose where to dwell according to the distribution coefficient. Synthetic drug design must take into account the importance of having a distribution coefficient that will allow transport in both aqueous blood and through organic membranes. Choosing a Solvent System Ether-water is a good choice for a solvent system. One important aspect when choosing a solvent system for extraction is to pick two immiscible solvents. Some common liquid/liquid extraction solvent pairs are water-dichloromethane, waterether, water-hexane. Notice that each combination includes water. Most extractions involve water because it is highly polar and immiscible with most organic solvents. In addition, the compound you are attempting to extract, must be soluble in the organic solvent, but insoluble in the water layer. An organic compound like benzene is simple to extract from water, because its solubility in water is very low. However, solvents like ethanol and methanol will not separate using liquid/liquid extraction techniques, because they are soluble in both organic solvents and water. There are also practical concerns when choosing extraction solvents. As mentioned previously, the two solvents must be immiscible. Cost, toxicity, flammability should be considered. The volatility of the organic solvent is important. Solvents with low boiling points like ether are often used to make isolating and drying the isolate material easier. If ether is used (bp = 35 °C) then evaporation to collect the solid is fast. Identifying the Layers The Drop Test. One common mistake when performing an extraction is to mix-up the layers and discard the wrong one. The densities of the solvents will predict which solvent is the top or bottom layer. In general, the density of nonhalogenated organic solvents are less than 1.0 g/mL and halogenated solvents are greater than 1.0 g/mL. One common solvent pair is dichloromethane and water. The density of dichloromethane is 1.325 g/mL and water is 1.000 g/mL. Dichloromethane is more dense that water; therefore, dichloromethane will be the bottom layer and water will be the top layer. Table 6.1 lists the densities of some extraction organic solvents. Solvent hexane ether toluene water dichloromethane chloroform Density (g/mL) 0.695 0.708 0.867 1.000 1.325 1.492 Table 6.1: Common extraction solvents listed by density. (For a complete list of physical properties of some common organic solvents, please see the table located in the front of your laboratory notebook.) Although the density is the physical property that determines which layer is on top or bottom, a very concentrated solute dissolved in either layer can reverse the order. The best method to avoid making a mistake is a drop test. Add a few drops of water to the layer in question and watch the drop very carefully. If the layer is water, 76 then the drop will mix with the solution. If the solvent is the mistaken organic layer, then the water drop will create a second layer. In general, this method can help determine the identity of the layer. However, it is still best to keep ALL the layers until the extraction is complete and your product has been isolated. Separating the Layers Once the two layers are mixed, you will need to separate them. You could separate the two layers by pouring off the less dense layer into a separate container. You would find that it is difficult to do this cleanly, however. With a water-ether mixture, you would undoubtedly end up with some ether left on top of the water or some water poured off with the top ether layer. To make the separation of two liquids in liquid/liquid extraction, chemists use a separatory funnel. Use a separatory funnel to separate the two layers. Figure 6.5: A Separatory Funnel. After making sure the stopcock at the bottom is closed (in the horizontal position), the complete reaction mixture including both aqueous and ether layers is poured into the separatory funnel. The lower aqueous layer is drained into a beaker or flask by opening the stopcock. Just as the interface between the two layers enters the stopcock, the stopcock is closed. The ether can then be drained out the bottom or poured out the top into a separate beaker or flask. However, since there are usually droplets of water containing inorganic salts clinging to the walls of the separatory funnel or floating in the ether, chemists often keep or place the organic layer in the separatory funnel and extract it with a volume of pure distilled water. Removing traces of unwanted materials this way is often called washing. Extraction and washing are not very effective unless the two layers are 77 Be careful adding the liquid to the separatory funnel. Remember to close the stopcock!! Washing mixed together vigorously to provide maximum surface contact between the two immiscible layers so that substances can be pulled or extracted from one into the other. To do this, the separatory funnel is stoppered. With one hand gripping the top of the funnel so that a finger holds in the stopcock, the separatory funnel is tipped upside down. Gently shake or swirl the funnel to mix the two layers. Open the stopcock with your other hand to relieve pressure that usually builds up from the vapor pressure of ether or another solvent. Vent often and point the funnel away from yourself and classmates while shaking the solution. Since extraction solvents typically have a very high vapor pressure (low boiling point), considerable vapor pressure is created while mixing the two layers. Several times during lab, separatory funnel caps have “popped off” due to built up vapors. Figure 6.6: The proper method to vent a separatory funnel. Microscale Extraction Thorough mixing is very important because the two solutions must be in contact with each other to allow the solute to be extracted into the second layer. Once the immiscible layers have been thoroughly mixed, with the funnel open to the atmosphere, drain the bottom layer into a clean Erlenmeyer by slowly turning the stopcock as described above. Each layer can be easily separated using this method. Again, several extractions should be performed to completely extract the materials. Pool the organic layers, evaporate the solvent, and your separated compound is left behind. For microscale separations, pipet layer separation is convenient and normally very little product loss is incurred. Since the two solvents are already in a reaction tube, instead of transferring the small volumes of solvent to another piece of glassware and ultimately losing product, the solvents can be mixed and separated directly from the reaction tubes. Use a Pasteur 78 pipet to gently mix the layers. This can easily be accomplished by gently drawing the liquid up and down with the pipet. Do not simply swirl the tube. This mixing method will not allow the two layers to mix properly and decreasing the success of the extraction. Once the layers are thoroughly mixed, use the pipet to draw up the bottom layer as shown in Figure 6.7 below. Pipet extraction for microscale separations. Water Layer Ether Layer Ether Layer Water Layer Figure 6.7: Microscale pipet extraction. The ether and water layers are now separated. Normally, two or more ether extractions would be completed to ensure the complete removal of the organic compound. Both the macroscale and microscale separations are typical examples of how liquid/liquid extraction can be used to separate water soluble inorganic materials from organic products. Finally, the ether or other organic solvent could then be evaporated, leaving the mixture of organic product with traces of starting material and by-products (often called the crude product). This can be purified by recrystallization or sublimation. Caution- do not discard the wrong layer. Emulsions An emulsion is a suspension of tiny droplets of one solvent mixed in the other. Emulsions are common in extraction because proper mixing is essential. In Italian salad dressing, an emulsion is desired to keep the water and oil mixed. Additives are added to the dressing in order to keep the two normally immiscible solvents miscible. In a liquid/liquid or solid/liquid extraction however, an emulsion will lead to a poor separation. Gentle shaking and swirling the separatory funnel is the best technique to avoid emulsions. However, if an emulsions occurs, there are several simple methods to destroy it. The first is time. Over time the layers will eventually separate. With a severe emulsion, you may not have time during a three hour lab period to wait. Another method is to add brine or salt water to the mixture. Since ether is less soluble in a highly ionic solution such as salt water, the ether and water will be forced to separate. This method works well with 79 Shake the mixture gently to avoid emulstions. Use salt water to remove emulsions. small emulsions. If you have a more difficult emulsion, separate the layers as much as possible and dry the organic layer with a drying agent. The water will be removed from the organic layer along with the drying agent. Subsequent extractions should proceed without further trouble. Drying Agents One significant problem with liquid/liquid extraction is that no solvent is COMPLETELY insoluble in another solvent. In practice, one additional step is usually carried out before evaporating the organic solvent: drying over anhydrous sodium sulfate or other drying agent. Drying a liquid might seem like a peculiar concept, since we normally think of all liquids as being wet. Drying an organic liquid in the organic lab has a special meaning to chemists. It means to remove all traces of water. Even water and hexane are slightly soluble in each other. After separating the two solvents, residual water will remain in the hexane or ether organic layer. This will remain and stick to the solid product when we remove the more volatile solvent. Therefore, chemists remove the water from the organic layer by adding an insoluble inorganic solid to the solution which will absorb the water, thus “drying” it. Granular anhydrous sodium sulfate is the drying agent most often used although other drying agents are also available. All of the inorganic solids work by reacting with the water to form hydrates, which is their preferred form if water is available. Na2SO4 Na2SO4.5H2O H 2O + sodium sulfate MgSO4 MgSO4.7H2O H 2O + magnesium sulfate CaCl2 Sodium sulfate is used most often in this course. + CaCl2.6H2O H2 O calcium chloride 2CaSO4 + [CaSO4]2.H2O H2 O calcium sulfate "Drierite" Figure 6.8: Hydrated complexes for some common drying agents. These compounds will associate or hydrate themselves with water. Table 6.2 lists some common drying agents along with their speed, capacity, and hydration. Table 6.2: Common drying agents. Drying Agent Sodium Sulfate Magnesium Sulfate Formula Na2SO4 Speed Medium Capacity † High Hydration † † 7-10 7 MgSO4 Fast High Calcium Chloride CaCl2 Fast Low 2 Calcium Sulfate (Drierite) CaSO4 Fast Low 1/2-2 † Capacity refers to the amount of water removed per given weight of drying agent. †† Hydration is the number of water molecules removed per molecule of drying agent. 80 These drying agents do not dissolve in the solvent they are “drying”. They may change somewhat, for example, sodium sulfate will clump together as it reacts with water, but they will remain solids in normal extraction solvents. This makes them easy to remove by decantation (pouring off) of the liquid or by gravity filtration. Usually the organic solvent will go from cloudy to clear in the process of being “dried”. You should be careful to remove all of these solid drying agents before solvent evaporation or you might think they are your product. When you take a melting point and the product doesn’t melt by 300°C, you probably have isolated your drying agent. Sodium sulfate is a widely used drying agent and the one predominately used in this course. It is relatively inexpensive and fast. It is recommended that the drying agent you choose be in a granular form. After the drying agent has removed the residual water, it is easier to remove large granular particles. Drying a solvent however, is not an exact science. An excess of drying agent should be used to ensure that all the water is removed. If the water remains after the materials are collected, it could interfere with the analysis. Add drying agent until there are no longer clumps of drying agent stuck to the sides or bottom of the flask. The drying agents should be free floating in the beaker, like snow. Do not be afraid to use too much. There are many other choices for drying agents including molecular sieves and sodium metal. There are benefits and disadvantages to each one. Sodium, for example, is an excellent drying agent, however it violently decomposes in water to create NaOH and H2 gas and may ignite spontaneously. Therefore it should be used with caution and only when removing very small amounts of water. Many times a particular drying agent will work better than others in a certain situation. Use the Purification of Laboratory Chemicals as a guide when purifying organic compounds. Drying the solvent means to remove water. Acid/Base Extraction There are also three special cases of liquid/liquid extraction that are extremely useful for isolating and purifying amines, carboxylic acids and phenols. All three of these functional groups can be interconverted from non-ionic organic-soluble forms to water-soluble ionic forms by changing the pH. Amines: RNH2 + H+ Phenols: PhOH + OH- Carboxylic Acids: RCOOH + RNH3+ PhO- OH- H2O + RCOO- + H2O Solid/liquid or liquid/liquid extractions rely on the solubility of the solute to be extracted. In acid/base extraction, the molecule to be extracted is transformed so that we impose a new solubility on the molecule. One specific example is benzoic acid, an organic acid. Benzoic acid is soluble in most organic solvents including dichloromethane and ether. However, this acid can be easily deprotonated with base to give a charged ionic species that is readily soluble in water. COOH - COONa Base + (NaOH) Benzoic Acid + Sodium salt of Benzoic Acid 81 H2O Acid/base extraction is useful to separate acidic, basic and neutral components. Changing the pH of the aqueous phase changes the distribution coefficient. By converting benzoic acid to the sodium salt of benzoic acid, the solubility has drastically changed. Now the sodium salt is soluble in the water and will migrate to the water layer. Because the solvents chosen are immiscible in each other, the layers can be easily separated. Although the separation is complete, we no longer have benzoic acid. To obtain the original compound, the salt must be protonated with a strong inorganic acid. Once the benzoic acid is recovered by adding acid, it will precipitate in the water to provide a pure compound. This method works very well with mixtures of strong organic acids, weak organic acids, bases and neutral compounds. We can use the acid/base functionality to our advantage. By changing the pH of the aqueous phase in a liquid/liquid extraction, the distribution coefficient is drastically changed, thus pulling molecules into either an organic layer or aqueous layer at will. Carboxylic acids, phenols, and amines can be easily separated from neutral components. However, all other common functional groups are not affected by changes in aqueous pH and so they will always distribute between layers the same way because their distribution coefficient is unaffected by pH. Figure 6.9 details the reagents needed to separate benzoic acid, aniline and phenol. Acid/Base Equations COOH - Acid (NaHCO3) Benzoic Acid Benzoic Acid Sodium salt of benzoic acid + NH3 Cl NH 2 - Acid (HCl) Aniline (1) (HCl) NH 2 Base (2) (K2CO3) Aniline Protonated aniline OH - OH + O Na Strong Base Acid (NaOH) Phenol COOH + COO Na Weak Base (3) (HCl) Sodium salt of phenol Phenol Figure 6.9: Acid/Base Extraction Equations. Be sure you understand the chemistry before beginning your unknown separation. Acid/base extraction is one of the more difficult principles in organic chemistry to understand. The most straight forward approach to understanding this subject is to create a flow chart (mentally or on paper) to follow which species has been created and where the molecule resides. If you can imagine the molecule changing and moving to the appropriate layer, you will be able to complete the unknown separation very easily. Figure 6.10 is a detailed flow chart of the separation of a strong organic acid, a weak organic acid, an organic base, and a neutral component. If you can follow the steps involved below, the unknown extraction in this chapter will be much easier to understand. 82 OH COOH Building a flow chart can help you understand the acid/base separation. NH 2 Dissolve in organic solvent (ether or CH2Cl2) Extract with NaHCO3 (aq) water layer separate - COO Na organic layer + OH HCl NH 2 Extract with NaOH (aq) organic layer COOH separate water layer NH 2 - O Na + + NaCl HCl Extract with HCl organic layer separate water layer NH 3 Cl OH - + NaCl Evaporate Organic Solvent Allow to Dry NaOH NH 2 + NaCl + H 2O Figure 6.10: Flow Chart of an Acid/Base Extraction. Whether you use acid/base, solid/liquid or liquid/liquid, extraction is a useful organic tool to separate a mixture of compounds. From the early drugs that were extracted from trees and plants to modern day pharmacology, extraction is still used to separate and purify organic molecules. The following experiments demonstrate both acid/base extraction on a microscale and solid/ liquid extraction on a macroscale. 83 Sublimation Another easy and inexpensive purification technique is sublimation. It will be used as the final purification step in the isolation of caffeine from tea. P r e s s u r e liquid solid triple point vapor temperature Figure 6.11: A Phase Diagram. Sublimation is the phase change from a solid directly into the gaseous phase. Figure 6.11 is a typical phase diagram which can be used to determine whether a substance exists in the solid, liquid, or gaseous state at a particular temperature and pressure. The boundary lines between these three phases are determined experimentally for individual compounds. The phase changes of melting, boiling, and sublimation and the reverse processes of solidification (crystallization), and condensation for constant pressure systems are shown on the diagram. Note that one can also move between phases by changing pressure at constant temperature. You might draw the three lines for these constant temperature phase changes on the diagram. The triple point is the location where all three phases exist coincidentally. On heating, most solids we see in everday life melt, i.e. go from solid to liquid. However, a few, such as Dry Ice, change directly from solid to gas. If you have a compound that sublimes instead of melts, and you can resolidify the sublimed vapors back to a solid, you have the basis for a simple and efficeint purification method. Of course, this method only works if the impurities in the compound do not sublime. If a compound must be heated to a very high temperature to sublime at atmospheric pressure, it will likely decompose. As you can see by studying the solid/gas boundary line in Figure 6.11, the sublimation temperature will be lower at lower pressures. This behavior can be taken advantage of by carrying out the sublimation in a vacuum sublimation apparatus shown in Figure 6.12. In this case, sublimation can be carried out at a lower temperature. 84 Figure 6.12: A Simple Sublimation Apparatus. Sublimation can interfere with a melting point determination. To avoid sublimation during a melting point determination, seal the melting point capillary tube under vacuum as shown in Figure 6.13. Thick-walled Tubing to vacuum White Septa Heat Capillary Tube Here Sample Figure 6.13: Sealing a capillary tube under vacuum. First, insert a toothpick (Common Shelf) through the white septa found in your red kit. Take a melting point capillary tube, filled with the appropriate amount of sample and insert it in the whole created by the toothpick. Leave the open end of the capillary at the larger end of the septa. Attach the thick-walled vacuum tubing the septa and the house vacuum. Turn on the vacuum and allow the capillary to be evacuated. After evacuating the capillary, heat the top of the reaction tube with a Bunsen burner and seal off the capillary. Since the sealing of the tube involves a Bunsen burner (at Desks 76/77 and 132/133 only), please have your lab instructor assist you. Once the tube is sealed, obtain a melting point as usual. 85 Extraction Experiments Procedure 1 demonstrates the Microscale Separation of Acidic, Basic and Neutral Substances and Procedure 2 demonstrates the Macroscale Extraction of Caffeine from tea bags. Chem 35 students should do both liquid/liquid extractions (Procedure 1 & 2) in one lab period. Chem 36 students may do this too or do Procedure 2 in the second lab and carry out the sublimation of caffeine during the TLC Experiment (Ch 7), which is normally a shorter experiment. Procedure 1•• Microscale Separation of Basic, Acidic and Neutral Substances A mixture of equal parts of an unknown carboxylic acid, an unknown amine, and an unknown neutral compound is to be separated by the liquid/liquid extraction technique (see Figure 6.15). The carboxylic acid could be benzoic acid (1) or 3-toluic acid (2), the amine could be 4'aminoacetophenone (3) or 3'-aminoacetophenone (4), and the neutral compound 1,4dimethoxybenzene (hydroquinone dimethyl ether, 5), or 4,4'-dimethylbenzophenone (di-p-tolyl ketone, 6). Carefully follow the detailed directions for component extraction and isolation. Caution!! Note!! Cautions: Ether is extremely flammable and boils at a low temperature (your body temperature!) Work in your hood! No flames should be ignited in the lab during this experiment! Keep the ether in the hood and do the extraction in the hood. Every hood will have its own bottle of ether. The amines 3 and 4 are irritants. Wear gloves when doing this experiment or wash your hands frequently, particularly in the first step in which the amine is extracted. NOTE: Most volumes can be measured directly into the reaction tube using the calibration marks on the side of the tube as shown in Figure 6.14. Figure 6.14: Reaction Tube. Procedure: Obtain an unknown from your TA and record its number in your lab notebook immediately. Weigh the unknown plus the plastic “snap-top” vial, transfer the organic mixture to a reaction tube, and reweigh the empty “snap-top” vial to get the weight of starting mixture. It should be between 150 and 180 mg. If it is less, obtain another unknown from the stockroom. If it is more, remove some material and reweigh. O O C OH 1 C CH3 or OH 2 3-Toluic Acid m.p. 109°C Benzoic Acid m.p. 121°C O O C CH3 or 3 C H 2N CH3 4 H 2N 4'-Aminoacetophenone m.p. 106°C 3'-Aminoacetophenone m.p. 97°C OCH 3 O C 5 or OCH 3 1,4-Dimethoxybenzene m.p. 57°C CH3 6 CH3 4,4'-dimethylbenzophenone m.p. 92°C Figure 6.15: Possible unknowns for acid/base extraction. (Note: Compound 6 is not listed in older versions of Aldrich) 86 A. Separation of the basic component by extraction with acid: 1 4.0 Mix layers well with pipet; 3.5 3.0 2.5 2.0 1.5 1.0 2 1 4.5 4.5 4.5 4.0 4.0 3.5 3.5 3.0 Let layers separate O R OH R NH2 R H in ether 3.0 O 2.5 2.0 R 2.5 OH 2.0 in ether 1.5 1.0 0.7 0.7 0.5 0.5 5% HCl(aq) R H 1.5 Pipet into tube 2 1.0 0.7 R NH3+ Cl-(aq) Extract ether with water 0.5 Add 2 mL of ether to the solid mixture in the reaction tube which is now designated tube 1 by mrling with a wax pencil. Make sure the solid is completely dissolved before going on to the next step. Add 1.0 mL of 5% aqueous hydrochloric acid to tube 1. Mix the two immiscible layers vigorously by drawing up as much as liquid as possible into a Pasteur pipet so that the pipet (but not the pipet bulb!) is completely filled and then squirting it back into the reaction tube briskly. Do this between 15 and 20 times so that there is maximum mixing between the aqueous and organic layers, thus allowing extractable material to move from one layer to the other. Let the layers separate, draw off the lower layer using the pipet and place it in tared reaction tube labeled tube 2. Extract tube 1 with one 0.25-mL portion (6-7 drops) of water, again using pipet mixing. After separation, draw off the lower water layer and add it to tube 2. Now add 6-7 drops of ether to tube 2, pipet mix it thoroughly, and remove and discard the top ether layer (let it evaporate in a beaker in the hood). This is called backwashing and serves to remove any non-ionic organic material that might contaminate the contents of tube 2. Exactly what chemical species is in tube 2? Basic Component Question #1: What chemical species is in tube 2? B. Separation of the acid component by extraction with base: 1 1 3 4.5 4.5 4.5 4.0 4.0 4.0 3.5 3.5 3.0 3.0 Mix layers well with pipet; 3.5 3.0 O 2.5 R OH 2.0 1.5 Let layers separate in ether 2.5 1.5 R H 1.0 1.0 0.7 0.7 0.5 5% NaHCO 3 (Sat'd aq.) 2.5 in ether 2.0 0.5 R H O R 2.0 1.5 Pipet into tube 3 1.0 0.7 O-Na+(aq) Extract with 0.5 NaHCO3(aq) again. Add 1.0 mL of a saturated aqueous solution of sodium bicarbonate (approx. 5-10% NaHCO3) to tube 1. Pipet mix the layers thoroughly, allow the layers to separate completely and then draw off the lower layer into another tared reaction tube (tube 3). Add 6-7 drops (about 0.25 mL) of sodium bicarbonate solution to tube 1, mix the contents as before and add the lower layer to tube 3. Exactly what chemical species is in tube 3? Backwash the contents of tube 3 with 6-7 drops of ether and discard the ether wash just as was done for tube 2. 87 Acid Component Question #2: What chemical species is in tube 3? Isolation of the separated components: At this point you have the neutral component in ether in tube 1, the amine component as its water-soluble ammonium chloride salt in tube 2 and the carboxylic acid component, as its watersoluble sodium carboxylate salt in tube 3. The isolation of the neutral compound (Step C) is quite simple; anhydrous Na2SO4 is added to absorb water from the ether solution, then separated by decantation. Evaporation of the ether yields the solid neutral component. In Step D, the amine is isolated by converting its water-soluble ammonium form to the nonionic amine by adding the base K2CO3. The solid amine should precipitate and can then be filtered off. In Step E, the carboxylic acid is isolated by converting it from its water soluble sodium salt to the insoluble non-ionic acid form by adding HCl. The solid carboxylic acid that precipitates is filtered off and dried C. Isolation of the Neutral Component: 1 4.5 4.0 3.5 3.0 1 Mix with pipet; Let layers separate; Remove NaCl(aq); Add Na2SO4(anhyd.) 4.5 Cork, Shake occasionally 4.0 3.5 3.0 2.5 2.5 2.0 for 5 - 10 min Continued below 2.0 in ether 1.5 1.5 R H 1.0 1.0 0.7 0.5 Size O corks can be found on the Common Shelf. 0.7 NaCl (sat'd aq) To tube 1 add 1 mL of saturated NaCl solution, pipet mix, and remove the aqueous layer. If the volume of your ether layer has now dropped below 1.5 mL, add enough ether to make the total volume about 2 mL. Now add to this enough anhydrous sodium sulfate to fill the tube with solid up to the 0.5 or 1.0 mL mark. Cork with a size 0 cork and shake occasionally over a period of 5 to 10 min. This drying agent does not react with the product, but only absorbs the water from, i.e. “dries”, the ether. It will be washed off with ether after the drying process is finished. During the 10 min drying time, you can work on steps D and E, then return to this point. 1 4 4 4.5 4.5 4.5 4.0 4.0 4.0 3.5 3.0 Decant off ether 3.5 Evaporate Ether 3.5 3.0 3.0 2.5 2.5 2.5 2.0 2.0 2.0 1.5 1.5 1.5 1.0 1.0 1.0 0.7 0.7 0.5 0.5 0.7 0.5 Be careful not to get any drying agent into the final ether solution. Na2SO4 (anhyd.) 0.5 into tube 4 Na2SO4 (anhydrous) RH The neutral component is recovered by decanting (carefully pouring off) the ether from the solid drying agent into a tared reaction tube, tube 4. The drying agent in tube 1 is washed once or twice with additional small amounts of ether to ensure complete transfer of the product, the 88 ether washes being combined with the main ether extract in tube 4 by decanting. Do this carefully so that no solid sodium sulfate is transfered into tube 4. The used sodium sulfate should be allowed to dry in the hood and discarded in the waste bin. Set tube 4 in a beaker in the your locker and allow the ether to evaporate until the next lab period. Alternatively, if you have time, blow off the ether in the hood with a stream of nitrogen from a plastic pipet connected to the nitrogen line with a piece of Tygon tubing. Warm the tube in a beaker of warm water to speed up this process. Determine the weight and m.p. of the crude neutral compound and, if necessary, (re)crystallize it from methanol/water. The product is dissolved in approximately 0.5 mL of methanol and a few drops of water is added until the solution gets very slightly cloudy or turbid, indicating the solution is saturated. This process is best carried out while heating the tube in a hot water bath at 50°C. [Because the product melts at 58-60°C, it is obviously impossible to have crystallization occur above 58°C.] Allow the tube to cool slowly to room temperature and then cool it thoroughly in ice. The product is best isolated by removing the solvent using a Pasteur pipet with a square tip. Dry the sample thoroughly and determine the percent recovery, the melting point and thus the identity of your neutral compound. Do not hand in any products. Discard them in the proper recycling containers in the hooded shelves. Inspect your pipet for cracks on the end. D. Isolation of the Amine Component: 2 2 4.5 4.5 4.5 4.0 4.0 4.0 3.5 3.5 3.5 3.0 3.0 2.5 R 2.0 NH3+ Cl-(aq) 50% K2CO3(aq) 2.5 R NH2 Cork & shake ppct. in H2O 2.0 3.0 Pipet Filter Dry 2-5 days 2.5 2.0 1.5 1.5 1.0 1.0 0.7 0.7 1.0 0.7 0.5 0.5 0.5 1.5 To precipitate the basic component add base. 2 R NH2 Make the contents of tube 2 basic by adding small amounts (3 or 4 drops) of 50% potassium carbonate (aq). Test with litmus paper for a slightly basic pH of 8 to 10. Agitate to mix and then cork (Size O, Common Shelf) and shake for a minute. This will cause the amine to precipitate out. Cool the tube in ice for a few minutes and remove the solvent from the crystals with a Pasteur pipet (make sure pipet tip is square!) Wash them with a very small quantity of ice water and allow to dry in a beaker, on a watch glass or on a filter paper until the next lab. The percent recovery and melting point are determined and the amine thus identified. E. Isolation of the Carboxylic Acid Component: See next page. 89 E. Isolation of the Carboxylic Acid Component: Add acid to precipitate the acidic component. 4.5 4.5 4.5 4.0 4.0 4.0 3.5 3.5 3.5 3.0 2.0 R - + O Na (aq) 3.0 O Conc. HCl O 2.5 Question #3: Calculate the approximate amount of HCl needed to acidify the contents of tube 3. 3 3 3 Pipet Filter R OH ppct. in H2O 2.0 3.0 2.5 2.5 Dry 2-5 days 2.0 1.5 1.5 1.5 1.0 1.0 1.0 0.7 0.7 0.7 0.5 0.5 0.5 O R OH Assuming the NaHCO3 solution used to extract the acid in step B was 1M and using the information that concentrated HCl has a volume of 85.5 mL/mole, calculate roughly how much concentrated hydrochloric acid is needed to acidify the contents of tube 3. Then, by dropwise addition of concentrated hydrochloric acid, carry out this acidification, finally testing the solution with litmus paper to assure it’s acidic. (Be sure to mix thoroughly before testing the litmus paper, since in the process of transferring a drop onto litmus paper, you’re really testing the pH of the top of the solution in the tube!) An excess of hydrochloric acid does no harm. This acidification must be carried out with extreme care because much carbon dioxide is released in the process. Cool the tube in ice and remove the liquid by the pipet microscale filtration method (make sure pipet tip is square!) and discard the liquid. Add 0.75 to 1.0 mL of distilled water and a boiling stick to the tube and very cautiously heat it in a sand bath to bring most of the solid carboxylic acid into solution. (The water may need to reflux up the sides of the tube to dissolve all the crystals.) Allow the tube to cool slowly to room temperature and then cool it in ice. Remove the solvent from the crystals with a Pasteur pipet (make sure pipet tip is “square”, i.e., not chipped!) [The solubility of benzoic acid in water is 1.9 mg/mL at 0°C and 68 mg/mL at 95°C. 3-toluic acid is not much different.] Dry the crystals by spreading them out on a watch glass or filter paper in your locker for a few days. When thoroughly dry, determine the weight and percent recovery of the solid carboxylic acid. Determine the identity of your carboxylic acid by its melting point. 90 Procedure 2•• Isolation of Caffeine from Tea As discussed in the introduction, the extraction of organic compounds from natural products is widely used. In this experiment, caffeine will be extracted from hot tea bags. O 1. Steep in hot H20 CH3 N CH3 N Three Tea Bags Caffeine 2. Extract with CH2Cl2 O N CH3 N Heat 75 mL of water in a 250-mL beaker to boiling on a hot plate (located on the shelf above your bench). Remove the boiling water from the hot plate and using paper towels as a hot pad, drop in three tea bags and allow them to steep (soak) for seven to ten minutes. Steeping involves soaking, but not boiling in hot water. The water solution is decanted into a 125-mL-Erlenmeyer flask and an additional 15 mL of hot water is added to the tea bags. This water solution is also decanted into the original tea extract, and the tea bags are gently pressed with a spatula to remove as much of the excess water as possible. The dark brown/red solution which results has a total volume of between 80 and 85 mL. If it is less than this add enough saturated aqueous sodium chloride solution to make it up to this volume. The solution must be allowed to cool to room temperature and is then placed in the 125 mL separatory funnel from your blue Kimble glassware kit. Add 15 mL of dichloromethane to the separatory funnel. The two liquids form two separate layers with the dichloromethane layer (initially colorless) on the bottom. The solutions are shaken gently together for approximately two minutes (Review Figure 6.6) and then allowed to separate. A glass stirring rod can be used to break up the emulsion. If the emulsion persists, try adding some more saturated salt solution. Using a 3" iron ring to hold the separatory funnel, the bottom layer is removed (drained out through the stopcock) along with a small emulsion layer into a clean 125-mL-Erlenmeyer flask. The extraction of the tea solution is repeated a second time by gently shaking with another 15 mL of dichloromethane and adding this to the first extract. The remaining tea solution can be discarded down the sink drain. The resulting dichloromethane extract solution is a pale greenish yellow with some undissolved brown liquid spots floating on the surface. Add enough anhydrous sodium sulfate to dry the solution. Swirling absorbs any water and brown liquid droplets. After drying the extracts over the anhydrous sodium sulfate for 5 to 10 min, the solution is decanted from the sodium sulfate into a 50-mL Erlenmeyer flask. Rinse the sodium sulfate with an additional 5-10 mL of dichloromethane and add this to the previous extract solution. Label this flask with your name and desk number and set it in the back of your hood until the next lab period (or, if the volume is rather small before you leave, in your locker) by which time the dichloromethane will have evaporated, leaving behind greenish crystals. Remove the emulsion by adding salt water or using a drying agent. Add a few drops of dichloromethane to the crystals and gently warm to dissolve them. Carefully transfer the solution to a 13 x100-mm test tube (not a reaction tube). Rinse the flask with additional drops of dichloromethane until you are sure the caffeine has been thoroughly transferred or washed into the test tube. Set the test tube in a rack or beaker and allow the solvent to evaporate in your locker until the next lab period. Or, if you have time, place a boiling stick in the tube and gently heat the dichloromethane solution until it boils. Once all the dichloromethane has evaporated or boiled off, insert the test tube into a hot sand bath (remove the boiling stick). The caffeine will sublime up the sides of the tube over a period of 20 to 30 min, forming long needles that attach to the cool walls at the top of the tube. Remove the test tube from the sand bath and lay it on the bench to cool. Reach in with a micro spatula and pull out one or two pure crystals and take a melting point. Tap the rest of the sublimed crystals onto a tared sheet of weighing paper and determine the weight of sublimed caffeine isolated. 91 Careful - only pull out the purified crystals. Final Report Final Report In your RESULTS AND DISCUSSION section, answer the questions that are embedded in the procedure for part 1 about what species are present in each of the extracts. (There are three questions that are required in the text.) Show explicitly the calculation of the amount of concentrated HCl required to neutralize the base in tube 3. Assuming each component was onethird of your starting mixture’s weight, calculate the percent recovery of each. Give the identity of each of your three compounds based on the melting point. Be sure to give your unknown number. Discuss liquid/liquid extraction. as a compound separation method; what functional group classes is it good for? Use the point distrubution on the grade sheet as a check list to see if you have included the required information. Post-Lab Question PostLab Question Using the formula below, determine whether or not it is preferable to make a single extraction with the total quantity of solvent or to make several successive extractions with smaller portions of the solvent. The formula to determine the fraction extracted into B = 1 1 + VB VAn K n where K is the distribution coefficient VA is the volume of the solvent A VB is the volume of the solvent B n is the number of extractions. Suppose a system consists of 50 mg of organic compound dissolved in 1.00 mL of water (solvent A). Compare the effectiveness of one 1.5-mL extraction versus three 0.5-mL extractions with ether (solvent B) where the distribution coefficient, K, is equal to 10. The answer gives you a fraction of the amount of organic compound extracted into solvent B, ether. How much material (in mg) is left in the water layer? Discuss which extraction method is preferable; one or three extractions? Lab and PostLab Sections - Final Report: Accuracy and completeness OBSERVATION/DATA of section 12 RESULTS/DISCUSSION Overall organization, readability, completeness 13 Calculations and answers to questions in experimental procedures 25 Correct identity of the three unknown compounds in your mixture. 24 Melting point accuracy of caffeine 8 Discussion of extraction as a compound separation method 6 PostLab Questions 12 Total for Final Report 100 92