States of matter Solid: Fluid: • Liquid
advertisement
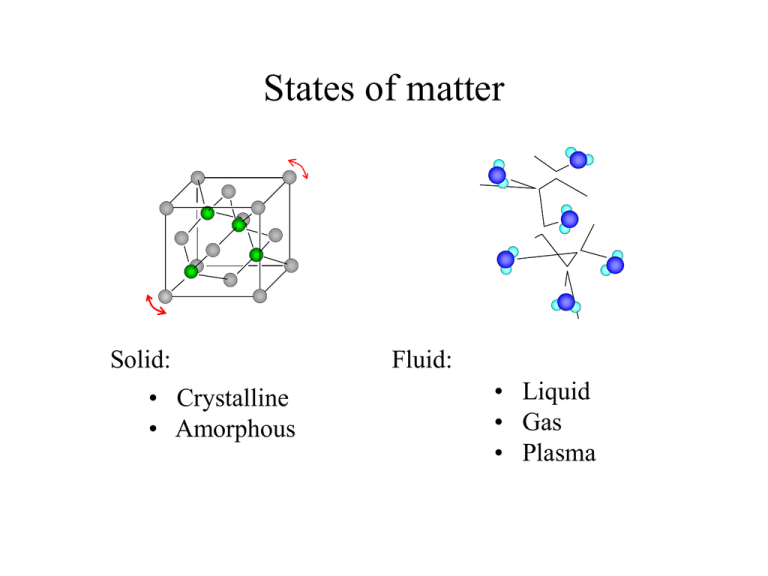
States of matter Solid: • Crystalline • Amorphous Fluid: • Liquid • Gas • Plasma internal interaction zz z yz xz zx yx xx x zy xy yy y In a medium, a set of parameters leading to the forces exerted on an infinitesimal cube element within the medium, is called the stress tensor. ij dFij dA where is the i-th scalar component of the force exerted on the j-th wall of the cube and dA is the area of one wall. The SI unit of stress is the pascal (Pa). Note: Only six independent components. deformation z dyz dzz dxz The deformation is described by a strain tensor dzy dyy y dxy dxx x dyx dzx ij d x i j da where d(xi)j is the displacement of the j-th corner in the i-th direction, and is the size of the cube (initial). Hook's law Within certain limits, the differential change in stress, caused by external forces exerted on the medium, is a linear function of the differential strain. ij ijkl kl kl or ˆ ˆ ˆ The proportionality tensor is called a modulus. tension z The external forces, applied along a single line to two opposite sides of the rod, cause a uniform stress dF dL dF dL Y zz zz Y A L Coefficient Y is called Young's modulus. L We can often approximate a finite change in the related quantities using the above y differential relation x -dF F Y L A L compression (uniaxial pressure) z The external forces are applied along a single line to two opposite sides F F L zz Y zz Y A L L L The nonzero component of compressive stress is called uniaxial pressure (P) y x -F dF P zz dA Shear stress z dy Tangential external forces applied to two opposite sides of the object cause a shear stress dF h d -dF x y dy dF yz S yz S S d h A Coefficient S is called the shear modulus. Comment 1. Fluids in rest do not create shear stress. Comment 2. The occurrence of a velocity dependent stress in a moving fluid is called viscosity. Hydrostatic pressure z dF dF dF y dF x dF Under hydrostatic pressure, all shearing components of the stress are zero and all compressive components of stress are equal. dP xx yy Hook’s law: dV dP B V dF zz A fluid at rest F0 F0 W field, pressure in PIn (h )agravitational A F0 h P0 fluids dependsh on the pressure created by an external in P0 A force gAdhand ' the depth h the fluid 0 W P0 gdh ' A Ph P0 gh for uniform density: P(h) h gdh ' gh 0 F(h) 0 Pascal's principle A change in the pressure applied to an enclosed (incompressible) fluid is transmitted undiminished to every portion of the fluid. F1 A2 F1 F2 Hydraulic Press: A1 A1 A2 y Archimedes' principle dA2 dBy P1 dA1 cos 1 P2 dA2 cos 2 P1 dA P2 dA g h dA gdV 2 dA 1 dA1 A body submerged (partially or completely) in a fluid is buoyed up with a force equal in magnitude to the weight of the fluid displaced by the body Ideal fluid • nonviscous - there is no internal friction; • flows steadily - at any point, the velocity of the fluid does not depend on time; • incompressible - its density does not depend on pressure; • irrotational - does not produce vortices When the rate of flow is small (laminar flow), many fluids can be approximated by the ideal fluid. Bernoulli's equation v2 y2 A2 dx2 v1 y1 A1 dx1 v 22 v12 gy 2 P2 gy1 P1 2 2 For in ideal fluid, the sum of the pressure, the kinetic energy per unit volume, and the potential energy per unit volume has the same value at all points along a streamline. from the work-energy theorem: A 2dx 2 v 22 A1dx1 v12 gA dx y P2A2dx 2 gA 2dx 2 y 2 1 1 1 P1A1dx1 2 2 Thermal contact Two systems are in thermal (diathermic) contact, if they can exchange energy without performing macroscopic work. This form of energy transfer (random work) is called heat. Mechanisms of Heat Transfer 1. Thermal Conduction law of thermal conduction: dQ T kA dt x more precisely : A dx dQ kA T dt Mechanisms of Heat Transfer 1. Convection natural convection: resulting from differences in density forced convection: the substance is forced to move by a fan or a pump. The rate of heat transfer is directly related to the rate of flow of the substance. dQ = cTdm Mechanisms of Heat Transfer 1. Radiation Energy is transmitted in the form of electromagnetic radiation. Stefan’s Law dQ AeT4 dt = 6 10-8 W/m2K A – area of the source surface e – emissivity of the substance T – temperature of the source E B Zeroth law of thermodynamics Thermal Equilibrium: If the systems in diathermic contact do not exchange energy (on the average), we say that they are in thermal equilibrium. If both systems, A and B, are in thermal equilibrium with a third system, C, then A and B are in thermal equilibrium with each other. Temperature We say that two systems in thermal equilibrium have the same temperature. (Temperature is a macroscopic scalar quantity uniquely assigned to the state of the system.) T 273.16 K lim P P3 0 P3 Gas Thermometer h T3 = 273.16 K is the temperature at which water remains in thermal equilibrium in three phases (solid, liquid, gas). The Celsius scale and, in the US, the Fahrenheit scale are often used. TC T 273.15 ; TF 9 TC 32 5 Thermal expansion For all substances, changing the temperature of a body while maintaining the same stress in the body causes a change in the size of the body. D dD l dl linear expansion: dl = ldl The proportionality coefficient (T) is called the linear thermal expansion coefficient. volume expansion: dV =VdV The proportionality coefficient (T) is called the volume thermal expansion coefficient.