Properties of Water Polarity and Hydrogen Bonding
advertisement

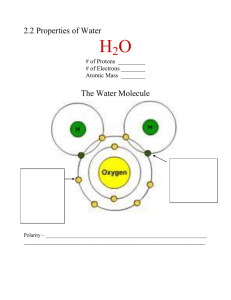
Date _____ Properties of Water Name _______________ Polarity and Hydrogen Bonding 1. If there are 600 water molecules in a glass. What is the total number of hydrogen atoms in the glass? What is the total number of oxygen atoms in the glass? _____________________________________________________________ _____________________________________________________________ 2. Why is water considered to be a polar molecule? _____________________________________________________________ _____________________________________________________________ 3. Explain why the oxygen atom in water has a negative charge. _____________________________________________________________ _____________________________________________________________ 4. Explain why the two pictures below are incorrect: _____________________________________________________________ _____________________________________________________________ 5. What is the name of the bond formed between water molecules? ___________________ 6. A student in biology class accidentally spills some vegetable oil on her favorite shirt. She tries to wash the oil off of her shirt with water but she is unsuccessful. Explain why it would be impossible to remove an oil stain with just water. Please talk about polarity in your answer. _____________________________________________________________ _____________________________________________________________ The diagram shows a salt crystal (NaCl) being dissolved in water. 7. In this mixture identify the solute and solvent. 8. Why are the water molecules attracted to Cl- ions and the Na+ ions in the salt? Date _____ Properties of Water Name _______________ Heat Capacity 9. As a substance is heated its molecules vibrate slower / faster. 10. Since water has a very high heat capacity the ocean temperature is very stable / unstable . 11. During a hot summer day the temperature of the water heats up much faster / slower than the temperature of the air. 12. Most animals in the ocean are cold blooded which means that their body temperature is equal to the temperature of the water. What would happen to the animals living in the ocean is that ocean temperature was to rapidly change? _____________________________________________________________ _____________________________________________________________ Cohesion and Adhesion 13. What is cohesion? ________________________________________________ _____________________________________________________________ 14. The object was gently placed on the surface of a body of water. Draw in the hydrogen bonds that allow this object that is denser to stay afloat. 15. How does waters polar nature account for its high surface tension? _____________________________________________________________ _____________________________________________________________ 16. Why does it hurt more to do a belly flop into a pool of water than dive in pencil style? _____________________________________________________________ _____________________________________________________________ If you lay a penny on a flat surface and add several drops of water on the face of the penny, the water will form a dome that never spills off of the side of the penny. 17. Explain how adhesion and cohesion prevent the water from spilling over the edge of the penny? Date _____ Properties of Water Name _______________ States of Matter 18. Does cold water (not frozen) sink or float in warmer water? Why? _____________________________________________________________ _____________________________________________________________ 19. Which state of water forms the most hydrogen bonds? 20. Which state of water forms the least hydrogen bonds. 21. What is the temperature range where water will remain a liquid? 22. At what temperature must you cool water to allow for a maximum of hydrogen bonding to take place? 23. Why do cracks in pavement widen over time to become pot holes during the winter? _____________________________________________________________ _____________________________________________________________ 24. The average cell is 65% liquid water. Explain why cells usually die when the water inside of them becomes frozen? _____________________________________________________________ _____________________________________________________________