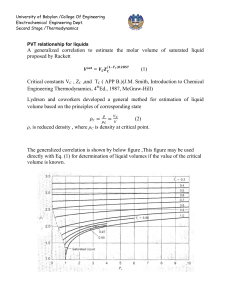
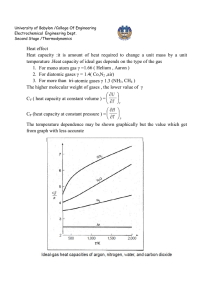
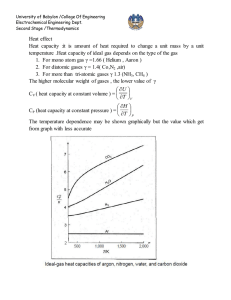
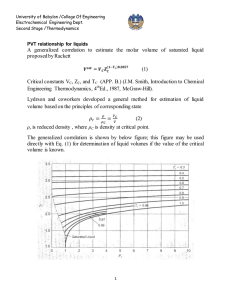
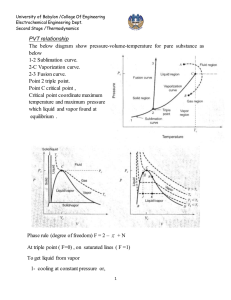
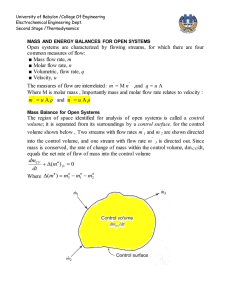
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics
advertisement
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics CUBIC EQUATIONS OF STATE If an equation of state is to represent the PVT behavior of both liquids and vapors, it must encompass a wide range of temperatures and pressures. Yet it must not be so complex as to present excessive numerical or analytical difficulties in application. Polynomial equations that are cubic in molar volume offer a compromise between generality and simplicity that is suitable to many purposes. Cubic equations are in fact the simplest equations capable of representing both liquid and vapor behavior. 1-The van der Waals Equation of State The first practical cubic equation of state was proposed by J. D. van der waals for gases in 1873: RT a P 2 V b V RTV 2 a (V b) P (V b)V 2 PV 3 (bP RT )V 2 aV ab 0 (1) Where 27 R 2TC2 RTC a b 64 PC 8PC PC and TC are critical pressure and temperature which are listed in App. B( J.M. Smith ,Introduction to Chemical Engineering Thermodynamics,4th Ed.,1987 McGraw-Hill) 2- Redlich-Kwong Equation For gases and liquids P RT a 12 V b T V (V b) (2) 0.42748R 2TC2.5 a PC b 1 0.08664 RTC PC University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics When multiply Radlich-Wkong equation by (V – b) / P gives below equation for vapor volume calculation V b RT P aV b T PV (V b) 12 To solve this equation use computer program, and for iteration we write: Vi 1 aV b RT b 12 i P T PVi (Vi b) The initial value V0 is provided by ideal gas equation (V0 = RT / P) Equation (2) is put into standard polynomial form and this can be used for liquid volume calculation V3 RT 2 bRT a ab V (b 2 )V 0 12 P P PT PT 1 2 Iteration can be written 1 RT 2 ab Vi 1 Vi 3 Vi c P PT 1 2 bRT a c b2 P PT 1 2 The initial value, take V0 = b. 3- Generalized Redlich-Kwong Equation When multiply Redlich-Kwong equation by (V / RT) PV RT V a V 12 RT V b RT T V (V b) RT V a V Z 12 V b T V (V b) RT Z 1 a h 1 h bRT 1.5 1 h 2 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Where h b b bP h ZRT / P ZRT Elimination of a and b gives 1 4.9340 h Z 1 h Tr1.5 1 h h 0.08664 Pr ZTr Where Tr T TC Pr P PC Figure 1 for PV diagram showing three such isotherms. Superimposed is the "dome" representing states of saturated liquid and saturated vapor. For the isotherm T1 > TC (figure 2) pressure is a monotonically decreasing function with increasing molar volume. The critical isotherm (labeled TC figure 3) contains the horizontal inflection at C characteristic of the critical point. For the isotherm T2 < TC,(figure 4) the pressure decreases rapidly in the sub cooled liquid region with increasing V; after crossing the saturated-liquid line, it goes through a minimum, rises to a maximum, and then decreases, crossing the saturated-vapor line and continuing downward into the superheated-vapor region. Figure -13 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Figure -2- Figure -3- Figure -4- 4