Semester 1 Chemistry Final Review
advertisement
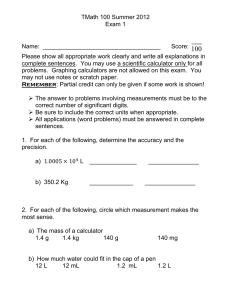
Semester 1 Chemistry Final Review Chapter 1 Describe basic research and explain why it is used. Give an example. What is the purpose of applied research, and how is it different from basic research? Describe extensive properties of matter, and give an example. Describe intensive properties of matter, and give an example. How can you describe the properties of all matter? What are the two properties that all matter will have in common. Describe an atom. What is a compound and how is it different from an element? How would you describe mass? Give one example of matter, give one example of something that would not be considered matter. Describe a physical change and give one example. Describe a chemical change and give one example. In what group can we find noble gases? What are they most known for? In a chemical reactions what are the elements or substances called that are combined? At the end of a chemical reaction what are the new compounds called? Describe the four states of matter. Give an example for each one. Describe a homogeneous mixture. How are elements arranged on the period table of elements? What is another name for a homogeneous mixture? How can you tell the difference between groups and periods in the periodic table? What is the main difference between a metal and nonmetal? How can you describe a metalloid? Chapter 2 Review What is the difference between weight and mass? Give an example Define volume, give an example using an object in the classroom. What is the formula for calculating density? How can you use the density formula to calculate mass? How can you use the density formula to calculate volume? Calculate the density of a piece of material with a mass of 5.03g that occupies 3.24mL of space. Calculate the mass of an object with a 3 volume of 55.1 cm and a density of 3 6.72 g/cm Calculate the volume of an object with a density of 0.824 g/mL and a mass of 0.451 g What is the purpose of dimensional analysis? Convert using dimensional analysis 10.5g to kg Convert using dimensional analysis 1.57km to m Convert using dimensional analysis 3.54ug to g Convert using dimensional analysis 1.2 L to mL How many significant figures? Explain your answer. 0.000305 kg Write in scientific notation 0.0006730 Write in scientific notation 50000.0 Write in decimal form 7.050 X 3 10 Write in decimal form 4.56 X ▪ -4 10