6. Functional Groups
advertisement

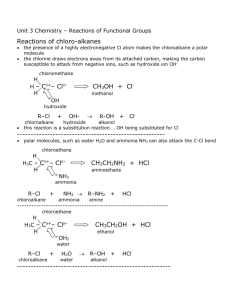
Functional Groups The bond, atom, or group of atoms which gives a molecule its specific properties is called its functional group. Eg The functional group of the alkenes is C = C; the functional group of the alkanols (alcohol) is – OH. Haloalkanes contain a halogen atom; F, Cl, I or Br (group 17 elements). These group 17 elements have replaced a hydrogen atom from an alkane Haloalkanes are not hydrocarbons The functional group is – X, where X is a halogen. The number of the carbon atom to which the halogen has been bonded must be given when naming haloalkanes, followed by the halogen and then the prefix indicating the number of carbons in the chain. The Alkanols are carbon chains containing one or more – OH group. The – OH group is sometimes called the ‘hydroxy’ group. Alkanols have the ending –ol. Note that each carbon atom has four bonds (valency 4), each oxygen atom has two bonds (valency 2) and each hydrogen atom has one bond (valency 1). Valency is the combining power of an atom. Many alkanols can be oxidised (slowly) by atmospheric oxygen and (quickly) by strong laboratory oxidants such as acidified potassium permanganate (KMnO4) or acidified potassium dichromate (K2Cr2O7). The product of such processes is usually a carboxylic acid. Alkanols also undergo combustion reactions in air. They can be used as fuels Propanol (C3H7OH) is commonly represented by the molecule propanol which has the structural formula Another isomer of propanol called propan-2-ol is commonly represented by the structural formula where the single lines between the atoms represent single covalent bonds. The carboxylic acids are another example of a homologous series. These compounds contain the functional group – COOH at the end of the chain. When naming a carboxylic acid, add the ending –oic acid to the alkyl prefix These acids have typical acid properties. They are classified as weak acids. ethanol + oxygen C2H5OH(aq) +O2(g) ethanoic acid + water. CH3CO2H(aq) + H2O(l) Complete the revision questions page 183 (29, 30) Carboxylic acids react with alkanols to form esters. Esters are a group of compounds which give the pleasant ‘fruity’ smell to various fruits. This is called a dehydration or esterification reaction. Concentrated sulfuric acid is used to link the alkanol and carboxylic acid together by removing a water molecule. H2SO4 CH3OH + CH3COOH methanol + ethanoic acid CH3COOCH3 + H2O methyl ethanoate + water When naming an ester, the alkanol (alkyl) name is given first, followed by the acid part. The –oic ending of the acid is replaced with –oate. Work 184 through the Sample Problem page The amines have the –NH3 functional group and are derived from ammonia, NH3. They are named by adding the suffix amine to the alkane group to which the NH2 has been added They are found in many natural and synthetic drugs. Used in the manufacture of antibiotics, analgesics and tranquilisers. Alchohol, carboxylic acids, esters on line multiple choice Complete the revision questions page 185 (31 – 39)