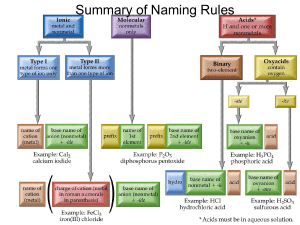
Meguerdijian Saro Meguerdijian Writing 340 Section 66801
advertisement

Meguerdijian 1 Saro Meguerdijian Writing 340 Section 66801 Assignment: Illumin Magazine Paper 3 May 2013 Imagining Chromium: The Diversity of Applied Chromium Chemistry Abstract Chromium has four main abilities: protecting, oxidizing, catalyzing, and manipulating electromagnetism. From stainless steel, to dyes and polyethylene (PE), to cassette tapes, engineers have found a variety of applications for chromium to improve society. However, hexavalent chromium (Cr(VI)) groundwater pollution continues to be a major public concern. Future developments in applied chromium chemistry include innovations in PE productions, economical treatment of Cr(VI), new chromium-containing metal alloys, and novel biomedical implants. Key words: chromium, hexavalent chromium, stainless steel, Nozaki-Hiyama-Kishi reaction, Phillips catalyst, polyethylene, dyes, pigments, chromium dioxide. Author bio: Saro Meguerdijian is a student majoring in Chemical Engineering (Petroleum Engineering Emphasis) with a minor in Business Law and plans to graduate in May 2015. He independently conducted research on treating hexavalent chromium in California’s groundwater, resulting in a potential treatment concept for which he was awarded the status of 2010 Siemens Competition Semifinalist. His interests include: the hydrocarbon industries, applied chromium chemistry, and computational modeling. Email: smeguerd@usc.edu Meguerdijian 2 Introduction Engineers often appear to be extreme specialists. From petrochemical pipe corrosion engineers to water-soluble polymer experts with an emphasis on fraccing, if one can imagine the engineer, he or she probably exists. Extreme specialization may be a consequence of engineers’ training and desire to dominate a niche in industry, but one should keep in mind that engineers are more than specialists: engineers are problem solvers. Sometimes, they use the same natural phenomenon to solve many different problems. Getting a clock to tick mechanically at fixed intervals is not so different from making a car’s wheels spin reliably. The concept of controlled rotation has been applied to both, albeit with widely varying results. Similarly, applied chromium chemistry – the study and application chromium’s properties – have snuck into nearly every aspect of life, usually by the initiative of some inventive engineer. “Inventive” is the key word here: chromium can only do so much. It may be extremely versatile (as the reader will soon see), but chromium has basically four main “abilities” that engineers use: protecting, oxidizing, catalyzing, and manipulating electromagnetism. These abilities, like the primary colors, may be limited in number but have a virtually infinite number of degrees and variations. Engineers harness these abilities in numerous applications to help make society safer, happier, and more economical. However, every science has its drawbacks, and applied chromium chemistry is no exception. Hexavalent chromium is widely useful but highly toxic, and many have suffered from excessive exposure. Fortunately, the other abilities of chromium more than compensate for this drawback. Meguerdijian 3 Ability #1: Protecting The modern explosion in the demand for chromium can be largely attributed to Harry Brearley, a British metallurgist. While experimenting with gun-barrel erosion during World War I, he discovered a type of steel that did not rust [1]. This alloy, or mixture of two or more metals, became known as “stainless steel.” Brearley essentially created an entire industry – the stainless steel industry – with his discovery. Society has greatly benefited from the discovery of stainless steel, as demonstrated in the ubiquity of the alloy in cutlery, buildings, and other applications. Stainless steel production depends on chromium for the steel’s protective properties [2], creating a major demand for chromium. Over $2 billion of chromium products were imported and exported by the United States in 2009, the vast majority of which is attributable to the stainless steel industry [3]. Chromium is clearly big business, largely due to stainless steel. The economic importance of stainless steel and its key component, chromium, is based upon chromium’s ability to protect. Perhaps surprisingly, pure chromium is a highly reactive metal. Chromium happens to be exceptionally “greedy” for particles called “electrons.” As a result, chromium often grabs electrons from generally corrosive substances (a process known as “oxidation”) and forms a thin, protective layer on its surface [2]. This layer protects the stainless steel from corrosive materials, increasing the steel’s usable life. The types of stainless steel include ferritic, austenitic, duplex-austenitic-ferritic, and precipitation hardening [2]. Ferritic steels are have high chromium content and so are more useful in applications requiring high corrosion resistance e.g. hot water tanks. Tough and corrosion resistant, austenitic stainless steels are used in the chemical industry and home appliances. Duplex-austenitic steel is stronger than austenitic steel and finds applications in desalination and the petrochemical industry. Precipitation-hardening steels contain a variety of metals, including chromium, and are known for their high strength and ability to be produced Meguerdijian 4 with precise dimensions, resulting in their use in the aerospace industry. Stainless steel has numerous uses, all of which depend upon chromium’s ability to protect. Chromium’s ability to protect is applied on a more personal level in chromium-alloy implants. Chromium alloys have been used in pulmonary stents, hip implants, and orthopedic implants [2, 4-6]. A pulmonary stent can be used to reinforce a blood vessel transplant when a vein is used to replace an artery. Since veins are less able to resist high blood pressures than arteries, a chromium-cobalt mesh can be used to support the vein without contacting the vein’s interior [5]. Another type of stent designed by Boston Scientific consists of a platinumchromium tube that medicates the patient as blood flows [4]. Called a “drug-eluting stent,” these tubes allow patients to lead normal lives during treatment, restoring patients’ sense of independence while ensuring that they continually have a proper level of drugs in their blood. The biocompatibility which permits these innovations emerges from chromium’s protective ability. Still, there is considerable controversy over metal-on-metal bone implants because of their ability to release metal ions into a patient’s bloodstream [6]. While the result on patient’s health is not well understood, litigation has resulted from perceived dangers associated with emissions of chromium and other metals from implants [6]. While implants are often made of chromium-containing alloys, other applications require coating a metal with a layer of chromium. A chromium-coated metal (called a “plated metal”) is more resistant to wear and corrosion, allowing the metal to be used for longer periods of time and under harsh conditions [7]. Chromium is generally applied to a metal surface through a process called “electroplating” in which an electric current is passed through a metallic object that one wishes to coat. The metallic object is first submerged in a liquid that contains chromium ions (chromium atoms that have lost electrons; usually hexavalent chromium [7]). When the electric current passes through the metal, it gives electrons to the chromium ions in the liquid. Meguerdijian 5 Upon accepting the electrons, the chromium ions become chromium metal and plate onto the metal. A basic visual introduction about chromium electroplating can be found at [8]. The resulting plated metal serves two main purposes in society: decorative and functional [7]. Decorative chromium electroplating produces the familiar lustrous chromium finish and is meant primarily for viewer’s pleasure. On the other hand, functional chromium electroplating (“hard chrome”) is used by industry for protecting equipment [7]. Ability #2: Oxidizing As discussed previously, chromium is a superb electron thief (“oxidizing agent”). The ability to protect is itself based on the ability to oxidize. Because of chromium’s wide use as an oxidizing agent for organic material, a separation section is included herein. Chromium’s oxidizing ability finds use in the preparation of various organic compounds. Organic compounds are those that contain the element carbon. These compounds consist of atoms bound to one another via bonds. A group of atoms bonded together is called a molecule. Chromium’s special role is in adding oxygen to organic molecules. At first glance, this may appear to be a banal ability – after all, what does adding an oxygen atom to a group of atoms based on carbon-carbon bonds achieve? A great deal! Many dyes are based on anthraquinone, an intermediate vital in the dye industry [9]. See Figure 1 to see how a special type of chromium called “hexavalent chromium” (Cr(VI)) takes hydrogen atoms from anthracene and replaces them with oxygen to make anthraquinone: Meguerdijian 6 Figure 1. Hexavalent chromium (Cr(VI)) oxidizes anthracene to form anthraquinone, a valuable base chemical for dye creation. Note that the Cr(VI) is very selective about which hydrogen atoms (H) it replaces with oxygen atoms (O). The unmarked intersections of lines represent carbon atoms, and the lines themselves represent single or double bonds. Cr(VI) oxidizes anthracene very selectively and with high conversion, which essentially means that the production of anthraquinone from anthracene is highly efficient [9]. Interestingly, the same oxidizing ability that allows Cr(VI) to facilitate this reaction also helps in wood preservation [10]. The wood industry used Cr(VI) oxide, a powerful oxidant, to protect wood from fungi and insects. A Cr(VI) treatment of wood may also make wood more fire resistant[11]. While Cr(VI) has found wide use in industry, from electroplating to dyes to wood preservation, Cr(VI) has also caused widespread fear because of its high toxicity, which is believed to result from its oxidizing ability [12] . In an episode publicized by the movie Erin Brockovich, the residents of Hinkley, California were exposed to Cr(VI) by poor disposal practices at Pacific Gas and Electric (PG&E) [13]. Cr(VI) had been used in the company’s cooling towers during the 1950s and 1960s and, after disposal in unlined ponds, contaminated the city’s groundwater. After much litigation, PG&E settled for $333 million with the residents in 1996 [13]. Many residents had become ill due to the Cr(VI) and, tragically, much Cr(VI) still remains in the city’s groundwater. Meguerdijian 7 The popularization of the incident and the State of California’s sense of responsibility for protecting its citizens led to the establishment of a Public Health Goal for Cr(VI) in groundwater [12]. Set at 0.02 parts per billion in drinking water, the standard is intended to specify an amount of hexavalent chromium exposure that will not result in harm. Hopefully, further action on this standard will help protect the people of California from further exposure and motivate national discussions between government, industry, and watchdog organizations over how to safely and economically implement Cr(VI) regulations. With the litigation over Sacramento’s groundwater’s Cr(VI) contamination just ensuing [14], effective public-private partnerships will be necessary. While Cr(VI) contamination is a concern domestically, it is also a concern internationally. Kellogg, Brown and Root (KBR) was hired to repair the Qarmat Ali water treatment plant in Iraq [15]. Sodium dichromate, a form of Cr(VI), had been used to maintain the plant’s pipes. KBR was later found to have knowingly exposed U.S. soldiers in Iraq to the sodium dichromate, potentially causing numerous illnesses. As a result, KBR was ordered to pay $85 million to those soldiers as compensation for their exposure. Litigation involving Cr(VI) exposure often is often expensive because of Cr(VI)’s extreme toxicity. A short note is necessary regarding the biological role of trivalent chromium (Cr(III)). Unlike Cr(VI), Cr(III) is considered an essential trace metal, though this has been called into question [16]. A hypothetical blood sugar regulator known as glucose tolerance factor (GTF) is believed to include Cr(III), which is the reason why Cr(III) is currently classified an essential trace metal. However, Cr(III) has not been found to perform any specific biological function in humans and has even been suspected of being carcinogenic [16]. The biological role of Cr(III) is highly uncertain but worth investigating due to its potential impact on human health. Meguerdijian 8 Ability #3: Catalyzing Chromium’s third major ability is that of catalysis. Certain forms of chromium can make reactions occur more quickly and under different conditions than they normally would. For example, chromia (chromium (III) oxide) does exceptionally well at removing hydrogen atoms from ethane to form ethylene (see Figure 2) [9]: Figure 2. Dehydrogenation of ethane to ethylene using a chromium catalyst. Through an intricate mechanism not shown here, the chromium catalyst causes the removal of two hydrogen atoms each bound to one of the carbon atoms, resulting in a double bond between the two carbon atoms. Ethylene and other carbon chains with double bonds are valuable industrial feeds, finding use in polymer and high-octane gasoline production [9]. Among the many reactions catalyzed by chromium, two merit special attention here: the polymerization of polyethylene (PE) and the Nozaki-Hiyama-Kishi reaction. These reactions were selected because PE production has major economic consequences [9], and the Nozaki-Hiyama-Kishi reaction presents a significantly different view of what is possible using chromium. The polymerization of PE essentially consists of joining a large number of ethylene molecules into a long chain. This chain of molecules is called a polymer [17]. PE-type polymers have a wide variety of uses. A non-exhaustive list: heavy-duty sacks, packing materials, construction, gellants for mineral oils, lubrication, toys, electrical fittings, bottles, wire and cable coating, and ink tubes for ballpoint pens [18]. Chromia-on-silica (silicon dioxide, or SiO2) catalysts were found to be an effective catalyst for joining ethylene molecules to one another to form long chains of PE [17]. These catalysts can create PE of high densities (known as high Meguerdijian 9 density polyethylene, or HDPE) [17] and low densities while maintaining a linear structure (linear low density polyethylene, or LLDPE) [9]. However, chromium catalysts used in the production of PE are known to give varying results dependent on minor differences in their preparation [9]. While this may initially seem like a disadvantage, this high sensitivity is actually a major advantage. It means that the properties of PE produced can be changed simply by modifying the chromium catalyst, without changing the raw materials. This allows for tremendous leverage in creating niche grades of PE. While PE polymerization provides exceptional value to society, the Nozaki-HiyamaKishi (NHK) reaction finds a more specialized role in the laboratory. The NHK reaction essentially consists of chromium (II) chloride acting in conjunction with nickel (II) chloride to attach a group of atoms to a special group containing a doubly-bonded oxygen (known as a ketone)[19]. See Figure 3: Figure 3. A highly simplified presentation of the Nozaki-Hiyama-Kishi reaction. Each R represents a different carbon-containing group of atoms. X represents chlorine, bromine, or iodine. The reader should focus on the transfer of the R1 group to the center carbon of the other molecule (ketone) and the addition of a hydrogen atom to the oxygen atom. The transfer of the R1 group is the main objective of the Nozaki-Hiyama-Kishi reaction. The NHK reaction in-and-of-itself has limited utility, but it is important as a tool for organic synthesis. Organic chemistry is essentially a “game” for scientists and engineers to use reactions such as this one to discover and manufacture new compounds, such as drugs or environmentallysafe chemicals. The NHK demonstrates chromium’s ability to be involved in this quest for new chemical discovery in a way other than polymerizing PE. Meguerdijian 10 Ability #4: Manipulating Electromagnetism Chromium’s fourth main ability consists of manipulating electromagnetic radiation (e.g. light) and magnetic fields. Chromium dioxide, a black, magnetic pigment, found widespread use for its ability to manipulate magnetic fields [10]. Chromium dioxide began to be used in tape recording in the late 1960s [20]. Chromium-dioxide pigments are less noisy than other pigments in cassette tapes and are the only pigments (as of 1990), that allow for thermomagnetic duplication [20]. Heating the chromium dioxide crystals in the tapes causes them to lose their magnetic orientation [21]. Once this occurs, the tapes can be magnetized in the desired format, permitting rapid duplication [21]. This became of commercial importance when bulk duplication of videotapes was needed prior to the CD and DVD [21]. Chromium’s ability to manipulate electromagnetic radiation, such as light, has found use in chromium-based dyes and pigments. Fundamentally, a dye or pigment absorbs certain wavelengths of light while reflecting others. As might be inferred by its name [22], chromium forms numerous colorful compounds. The colors of chromium pigments include various types of black, green, red, and yellow [10]. Notably, hydrated chromium oxide green reflects light in the infrared region, giving it importance in military camouflage paints [10]. One can essentially use chromium compounds to selectively absorb and reflect different wavelengths of light simply by modifying the compounds. Still, despite chromium’s utility in the creation of novel dyes and pigments, concern over the toxicity of hexavalent chromium may cause a decline in chromebased dye and pigment use. Solar cells are a promising area for chromium’s ability to manipulate light. Chromium has a history of use in manipulating light energy; in fact, chromium was used in early lasers and masers (which are like lasers, except that they emit microwaves instead of light) [23]. In meeting the National Academy of Engineering’s (NAE) Grand Challenge of making solar energy Meguerdijian 11 economical, lanthanum strontium chromium oxide (LSCO) and “black” chromium plating emerge as potential players [7, 24, 25]. Many metals that could be useful in solar cells are damaged via reactions with oxygen in air [26]. On the other hand, black chromium deposits and oxides such as LSCO are stable in air and so can be used in solar cells with minimum damage. Solar cells based on LSCO or black chromium could help make solar cells more economical. Future Progress The engineering applications of chromium are primarily limited by ingenuity. Although a few main abilities of chromium have been exploited in a variety of applications, the fundamental problems of applied chromium chemistry still remain unsolved. From the healthcare and environmental perspective, these problems include the bioactivity of trivalent chromium and effective remediation of chromium pollution. Trivalent chromium’s role or effect in the human body, if any, still remains unknown. An unequivocal answer to this long unsolved problem would have major immediate impacts in groundwater regulations and in medical recommendations concerning chromium. With respect to hexavalent chromium, the main problem is pecuniary: protecting the public from hexavalent chromium economically. If California’s Public Health Goal of 0.02 parts per billion for hexavalent chromium in drinking water becomes a legally enforceable limit, California’s water utilities’ compliance costs could rise significantly. Similarly, if federal levels of permissible hexavalent chromium in drinking water were reduced dramatically, water utilities nationwide would suffer greater compliance costs. Clearly, the safety of the public’s drinking water is vital, but the challenge will be to protect the public without placing excessive pressure on water utilities. In addition to healthcare and environmental issues, other important chromium-related problems include determining the oxidation number of chromium on the chromia-on-silica Meguerdijian 12 catalyst for the polymerization of polyethylene [9]. The scientific literature is rife with debate over this issue. A better understanding of chromia-on-silica could facilitate the discovery of new grades of PE or cost-saving methods of PE production. Further research could also lead to a better understanding of the properties of chromium, leading to new applications heretofore unimagined. With an increased understanding of chromium’s most basic behavior, chromium’s applicability could be extended. Additional areas of innovation in applied chromium chemistry will include the creation of dyes and pigments, medical implants, and extraterrestrial hexavalent chromium treatment. Dyes and pigments can be created to absorb various wavelengths or combinations of wavelengths. Chromium-based dyes are especially versatile in this respect. With growth in nanotechnology, engineers should explore methods to selectively control light absorption from the infrared to ultraviolet region using chromium nanoparticles. Because these nanoparticles can have sizes comparable to the wavelength of visible light, they may be able to manipulate light in novel ways. With the need for stronger and safer medical implants, chromium alloys should be thoroughly explored. Chromium-cobalt is currently “the standard” in biocompatible implants, though it is controversial in some instances [2,6]. However, Platinum-Chromium and other alloys may be able to provide alternatives that will help patients live healthier lives. The biocompatibility of many chromium alloys, stemming from their corrosion resistance, will ensure that chromium plays a major role in the field of metal implants. Non-metal implants may supplant chromium alloys if they are found to be more cost effective. However, given the high strength of chromium alloys, this will be challenging. Hexavalent chromium treatment costs may also become an important factor in extraterrestrial colonization. A report found that hexavalent chromium may be present in trace amounts in Martian dust [27]. If trace amounts of Cr(VI) are found in Martian dust, any human Meguerdijian 13 habitation of Mars would need to be strictly controlled for Cr(VI) exposure. Depending on the concentration of Cr(VI) in Martian dust, individuals inhabiting Mars may be burned simply via contact with the dust and potentially develop cancers. This threat could potentially thwart the creation of a long-term Mars colony for a considerable period of time. Assuming that Mars colonization would entail the creation of closed buildings for human habitation, highly efficient and effective air filtration and dust removal systems would be needed. Therefore, combining efforts toward creating air filtration systems for industrial settings and for Martian bases would be mutually beneficial. Meguerdijian 14 Works Cited [1] “HARRY BREARLEY, METALLURGIST, 77: Discoverer of stainless steel in 1916 dies – won Bessemer Medal for His Work,” The New York Times, p. 19, July 16, 1948. [2] P. Cunat, “Alloying elements in stainless steel and other chromium-containing alloys,” Euro-Inox The European Stainless Steel Development Association and the International Chromium Development Association, Paris, France, 2004. [3] J. F. Papp, “2009 Minerals Yearbook: Chromium [Advance Release],” U.S. Geological Survey, Washington D.C., Rep. 17.1-17.25, 2011. [4] Boston Scientific Corp. (2013, February 12). Promus Premier(TM) Everolimus-Eluting platinum chromium stent system receives CE mark approval [Online]. Available: http://news.thomasnet.com/companystory/Promus-PREMIER-TM-Everolimus-ElutingPlatinum-Chromium-Stent-System-Receives-CE-Mark-Approval-20003071 [5] R. Jasmer. (2013, February 1). A Little TLC Boosts Patency of Bypass Grafts [Online]. Available: http://www.medpagetoday.com/MeetingCoverage/STS/37154 [6] (2013). Kershaw, Cutter & Ratinoff LLP [Online]. Available: http://www.cobaltchromium-toxicity.com/ [7] N. V. Mandich and D. L. Snyder. “Electrodeposition of Chromium,” Modern Electroplating, 5th ed. Hoboken, NJ: Wiley, 2011, ch. 7, pp. 205-248. [8] Kosasihiskandarsjah. (2008, January 10). YouTube [Online]. Available: http://www.youtube.com/watch?v=RJ6j6YjJrHY [9] G. A. Olah and Árpád Molnár, Hydrocarbon Chemistry, 2nd ed. Hoboken, NJ: WileyInterscience, 2003, pp. 5, 40-41, 44-49, 53, 62, 64-65, 88-89, 114, 176, 325, 371, 431, 438, 445, 475, 482-483, 487-490, 496, 501-503, 519, 619-620, 639, 647, 701, 704, 749, 753758, 771-772, 778, 779, 782-784. [10] Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 2012, pp. 157-191. [11] “Chromium Chemicals,” Mutual Chemical Company of America, NY, 1941. [12] “Public health goal for hexavalent chromium (Cr VI) in Drinking Water,” Office of Environmental Health Hazard Assessment, CA, 2011. [13] P. Fimrite. (2010, November8). SFGate [Online]. Available: http://www.sfgate.com/green/article/Hinkley-water-tainted-by-chromium-6-spreading3167129.php Meguerdijian 15 [14] A. Hart. (2013, February 15). There’s still too much hexavalent chromium 6 in Sacramento’s tap water [Online]. Available: http://www.examiner.com/article/there-s-stilltoo-much-toxic-hexavalent-chromium-6-sacramento-s-tap-water [15] R. Trager. (2012, November 7). Royal Society of Chemistry [Online]. Available: http://www.rsc.org/chemistryworld/2012/11/us-national-guard-award-hexavalentchromium [16] D. M. Stearns, “Is chromium a trace essential metal?” BioFactors, vol. 11, pp. 149-162, 2000. [17] R. J. Young and P. A. Lowell, Introduction to Polymers, 3rd ed. Boca Raton, FL: CRC Press, 2011, pp.4, 152. [18] “POLYETHYLENE” in Condensed Encyclopedia of Polymer Engineering Terms. Oxford: Elsevier Science & Technology, 2001. [19] I. Thomé et al., “Trace metal impurities in catalysis,” Chem. Soc. Rev., vol. 41, pp. 979981, 2012. [20] H. Auweter et al., “Chromium dioxide particles for magnetic recording,” IEEE Trans. Magn., vol. 26, pp. 66-68, Jan. 1990. [21] G. R. Cole et al., “Thermomagnetic duplication of chromium dioxide video tape,” IEEE Trans. Magn., vol. 20, pp. 19-23, Jan. 1984. [22] D. Harper. (2012). Online Etymology Dictionary [Online]. Available: http://www.etymonline.com/index.php?term=chromium [23] R. D. Olt, “Synthetic Maser Ruby,” Appl. Opt., vol. 1, pp.25-32, 1962. [24] (2012). Engineering Challenges [Online]. Available: http://www.engineeringchallenges.org/cms/8996/9082.aspx [25] P. V. Sushko et al., “Multiband optical absorption controlled by lattice strain in thin-film LaCrO3,” PRL, vol. 110, pp. 077401-1 – 077401-5, Feb. 2013. [26] (2013, February 13). Phys.Org [Online]. Available: http://phys.org/news/2013-02lanthanum-chromium-oxide-energetic.html [27] Committee on Precursor Measurements Necessary to Support Human Operations on the Surface of Mars et al., “Chemical Environmental Hazards” in Safe on Mars: Precursor Measurements Necessary to Support Human Operations on the Martian Surface. Washington, D.C.: The National Academies Press, 2002, pp.29-32.