Unit 2 Test-Study Outline
advertisement

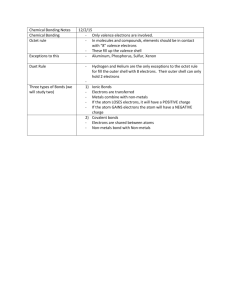
Name______________________________ Ms. Nersesian/Mrs. Bonewald Date________________ Period_____________ 8th Grade Honors Living Environment Unit 2 Study Outline This handout will serve as a resource to help guide you in studying for the Unit 2 Test. We encourage that you find a study method that works best for you. Use this page as a reference as you review class notes, handouts, quizzes, Castle Learning assignments, etc. In addition, there are excellent resources on the internet that you can use for supplemental review and reinforcement. Visit our teacher webpages for some suggestions on internet sources. As always, come to extra help and to our review sessions if you have any questions. Best of luck! 1. Inorganic Chemistry A. Biochemistry B. Matter vs. Energy C. Elements on the Periodic Table: i. Atomic Number (# of protons and # of electrons) ii. Atomic Mass (# of protons + # of electrons) iii. Valence Electrons iv. Be able to describe the difference between an atom, a molecule and a compound v. Be able to describe the difference between: Atom Ion Isotope vi. Valence Electrons determine an atom’s reactivity Max # of electrons in 1st energy level = 2 Max # of electrons in subsequent energy levels* = 8 vii. How do Lewis Dot Structures show us about valence electrons and bonding? viii. Covalent bonds Single bonds Double bonds Triple bonds ix. Ionic Bonds Oxidation/Reduction reactions LEO the lion says GER x. Water is polar → Hydrogen Bonding → Water’s Special Properties Ice floats on water Cohesion and Adhesion Transpiration Surface tension Water takes longer to heat up in the summer and longer to cool in the winter (Specific heat) Universal Solvent Name______________________________ Ms. Nersesian/Mrs. Bonewald Date________________ Period_____________ Solution Solvent Solute Aqueous solution D. pH Scale i. Acidic solutions ii. Basic solutions iii. Neutral solutions iv. Neutralization v. Be able to explain why buffers are important to living things. vi. How do indicators work? 2. Organic Chemistry A. Chemical Reactions i. Reactants ii. Products B. Organic vs. Inorganic C. Living things can convert inorganic molecules into organic molecules D. Why is Carbon THE main ingredient? E. Carbohydrates i. Monomer ii. Polymer iii. Ratio for Carbohydrates- 1:2:1 iv. Building blocks - Monosaccharides v. Isomers vi. Glucose vs. Glycogen vii. Disaccharides viii. Polysaccharides F. Dehydration synthesis vs hydrolysis G. Lipids i. Building blocks- fatty acids and glycerol ii. Hydrophobic vs. Hydrophilic iii. Unsaturated vs. Saturated H. Nucleic Acids i. DNA ii. RNA Name______________________________ Ms. Nersesian/Mrs. Bonewald iii. I. Proteins i. ii. iii. iv. v. Date________________ Period_____________ Building blocks- Nucleotides Functions Building blocks- amino acids Functional groups Polypeptides and peptide bonds The sequence of amino acids determine a protein’s shape. The shape of a protein determine its function. vi. Four levels of protein structure a) Primary b) Secondary c) Tertiary d) Quarternary vii. Denaturation viii. Enzymes- will be added after the Biochemistry test for the quarterly! 3. Test Taking Strategies A. Do not leave studying until the night before the test! Try to start studying a little every night so it does not seem so overwhelming! B. Bring pens, pencils, highlighters, graphing calculator, and straightedge (ruler) to the test. Being prepared will help save time and stress. C. Dress comfortably. Wear layers so you can be comfortable during the test. You want to eliminate all other distractions so you can focus on your exam. D. Wear a watch so you can manage how much time you have left for the exam. Our tests are 60 minute exams that are administered on our double period. (No breaks during the bell in between periods). E. Get a good night sleep the night before your test! Sleeping 8+ hours a night is VERY important. F. READ all of the information provided to you on your test. Make sure to read directions, diagrams, charts, background information, etc. Most importantly, READ WHAT THE QUESTION IS ASKING!! G. Underline or highlight key words. H. After you answer a question, go back and make sure it actually answered the question that is being asked! I. When answering multiple-choice questions, read all responses and cross out responses that you are certain are incorrect. Try to narrow it down to two choices and choose the response you feel best answers the question. There may be other tempting choices, so look for key words or distinctions between the choices to determine which one is the best answer. J. Short and concise responses are best. Sometimes writing too much can make an answer go from correct to incorrect. i. If the question says “state” or “identify” you do NOT need to write a full sentence. Just write your response on the line provided. ii. If the question asks you to “describe” or “explain” you must write in full sentences. Make sure to answer the question entirely! For example, if it asks you Name______________________________ Ms. Nersesian/Mrs. Bonewald Date________________ Period_____________ to DESCRIBE HOW the control group is treated from the experimental group, you must acknowledge both groups in your response! iii. If the question asks you to identify ONE or TWO factors, only write down the amount that is asked! If you write down three or four, the teacher will only grade you on the first two you have written. iv. Check your work for spelling! There is no excuses for misspelling words that are written in the question or information provided. We strive for excellence! One final thought… You are in an honors class which is going to be both a challenging experience and a rewarding one. Do your best to keep on top of your work and prepare for assessments well in advance. The more prepared, the more confident you will feel! These tips are general guidelines to help you in preparation for your first exam in Honors Living Environment. You are not limited to the information provided. Find what works best for YOU!